
Hepatitis C is an infectious disease caused by the hepatitis C virus (HCV) that primarily affects the liver; it is a type of viral hepatitis. During the initial infection period, people often have mild or no symptoms. Early symptoms can include fever, dark urine, abdominal pain, and yellow tinged skin. The virus persists in the liver, becoming chronic, in about 70% of those initially infected. Early on, chronic infection typically has no symptoms. Over many years however, it often leads to liver disease and occasionally cirrhosis. In some cases, those with cirrhosis will develop serious complications such as liver failure, liver cancer, or dilated blood vessels in the esophagus and stomach.

Ritonavir, sold under the brand name Norvir, is an antiretroviral medication used along with other medications to treat HIV/AIDS. This combination treatment is known as highly active antiretroviral therapy (HAART). Ritonavir is a protease inhibitor and is used with other protease inhibitors. It may also be used in combination with other medications to treat hepatitis C and COVID-19. It is taken by mouth. Tablets of ritonavir are not bioequivalent to capsules, as the tablets may result in higher peak plasma concentrations.

Dronedarone, sold under the brand name Multaq, is a class III antiarrhythmic medication developed by Sanofi-Aventis. It was approved by the FDA on July 2, 2009. Besides being indicated in arrhythmias, it was recommended as an alternative to amiodarone for the treatment of atrial fibrillation and atrial flutter in people whose hearts have either returned to normal rhythm or who undergo drug therapy or electric shock treatment i.e. direct current cardioversion (DCCV) to maintain normal rhythm. It is a class III antiarrhythmic drug. In the United States, the FDA approved label includes a claim for reducing hospitalization, but not for reducing mortality, as a reduction in mortality was not demonstrated in the clinical development program. A trial of the drug in heart failure was stopped as an interim analysis showed a possible increase in heart failure deaths, in patients with moderate to severe CHF.

Boceprevir is a protease inhibitor used to treat hepatitis caused by hepatitis C virus (HCV) genotype 1. It binds to the HCV nonstructural protein 3 active site.

Telaprevir (VX-950), marketed under the brand names Incivek and Incivo, is a pharmaceutical drug for the treatment of hepatitis C co-developed by Vertex Pharmaceuticals and Johnson & Johnson. It is a member of a class of antiviral drugs known as protease inhibitors. Specifically, telaprevir inhibits the hepatitis C viral enzyme NS3/4A serine protease. Telaprevir is only indicated for use against hepatitis C genotype 1 viral infections and has not been proven to be safe or effective when used for other genotypes of the virus. The standard therapy of pegylated interferon and ribavirin is less effective than telaprevir in those with genotype 1.

Sofosbuvir, sold under the brand name Sovaldi among others, is a medication used to treat hepatitis C. It is taken by mouth.

Simeprevir, sold under the brand name Olysio among others, is a medication used in combination with other medications for the treatment of hepatitis C. It is specifically used for hepatitis C genotype 1 and 4. Medications it is used with include sofosbuvir or ribavirin and peginterferon-alfa. Cure rates are in 80s to 90s percent. It may be used in those who also have HIV/AIDS. It is taken by mouth once daily for typically 12 weeks.

Ledipasvir is a drug for the treatment of hepatitis C that was developed by Gilead Sciences. After completing Phase III clinical trials, on February 10, 2014, Gilead filed for U.S. approval of a ledipasvir/sofosbuvir fixed-dose combination tablet for genotype 1 hepatitis C. The ledipasvir/sofosbuvir combination is a direct-acting antiviral agent that interferes with HCV replication and can be used to treat patients with genotypes 1a or 1b without PEG-interferon or ribavirin.

Nonstructural protein 5B (NS5B) is a viral protein found in the hepatitis C virus (HCV). It is an RNA-dependent RNA polymerase, having the key function of replicating HCV's viral RNA by using the viral positive RNA strand as a template to catalyze the polymerization of ribonucleoside triphosphates (rNTP) during RNA replication. Several crystal structures of NS5B polymerase in several crystalline forms have been determined based on the same consensus sequence BK. The structure can be represented by a right hand shape with fingers, palm, and thumb. The encircled active site, unique to NS5B, is contained within the palm structure of the protein. Recent studies on NS5B protein genotype 1b strain J4's (HC-J4) structure indicate a presence of an active site where possible control of nucleotide binding occurs and initiation of de-novo RNA synthesis. De-novo adds necessary primers for initiation of RNA replication.

Paritaprevir is an acylsulfonamide inhibitor of the NS3-4A serine protease manufactured by Abbott Laboratories that shows promising results as a treatment of hepatitis C. When given in combination with ritonavir and ribavirin for 12 weeks, the rate of sustained virologic response at 24 weeks after treatment has been estimated to be 95% for those with hepatitis C virus genotype 1. Resistance to treatment with paritaprevir is uncommon, because it targets the binding site, but has been seen to arise due to mutations at positions 155 and 168 in NS3.

Ombitasvir is an antiviral drug for the treatment of hepatitis C virus (HCV) infection by AbbVie. In the United States, it is approved by the Food and Drug Administration for use in combination with paritaprevir, ritonavir and dasabuvir in the product Viekira Pak for the treatment of HCV genotype 1, and with paritaprevir and ritonavir in the product Technivie for the treatment of HCV genotype 4.

Dasabuvir, sold under the brand name Exviera, is an antiviral medication for the treatment of hepatitis C. It is often used together with the combination medication ombitasvir/paritaprevir/ritonavir specifically for hepatitis C virus (HCV) type 1. Ribavirin may also additionally be used. These combinations result in a cure in more than 90% of people. It is taken by mouth.

Ledipasvir/sofosbuvir, sold under the trade name Harvoni among others, is a medication used to treat hepatitis C. It is a fixed-dose combination of ledipasvir and sofosbuvir. Cure rates are 94% to 99% in people infected with hepatitis C virus (HCV) genotype 1. Some evidence also supports use in HCV genotype 3 and 4. It is taken daily by mouth for 8–24 weeks.
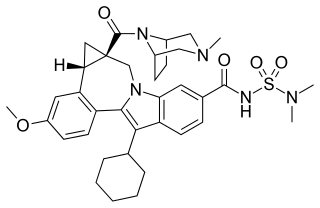
Beclabuvir is an antiviral drug for the treatment of hepatitis C virus (HCV) infection that has been studied in clinical trials. In February 2017, Bristol-Myers Squibb began sponsoring a post-marketing trial of beclabuvir, in combination with asunaprevir and daclatasvir, to study the combination's safety profile with regard to liver function. From February 2014 to November 2016, a phase II clinical trial was conducted on the combination of asunaprevir/daclatasvir/beclabuvir on patients infected with both HIV and HCV. Furthermore, a recent meta-analysis of six published six clinical trials showed high response rates in HCV genotype 1-infected patients treated with daclatasvir, asunaprevir, and beclabuvir irrespective of ribavirin use, prior interferon-based therapy, or restriction on noncirrhotic patients, IL28B genotype, or baseline resistance-associated variants

Gamal Esmat is a professor at Endemic Medicine and Hepatogastroenterology Department, Cairo University. He was vice president of Cairo University for Graduate Studies and Research.
Elbasvir/grazoprevir, sold under the brand name Zepatier, is a fixed-dose combination for the treatment of hepatitis C, containing elbasvir and grazoprevir. It is used to treat chronic hepatitis C virus (HCV) genotypes 1 or 4 infection in both treatment-naïve and treatment-experienced patients.

Daclatasvir/sofosbuvir is a two-drug combination for the treatment of hepatitis C. It is given as a single daily pill containing daclatasvir, a viral NS5A inhibitor, and sofosbuvir, a nucleotide inhibitor of the viral RNA polymerase NS5B.

Danoprevir (INN) is an orally available 15-membered macrocyclic peptidomimetic inhibitor of NS3/4A HCV protease. It contains acylsulfonamide, fluoroisoindole and tert-butyl carbamate moieties. Danoprevir is a clinical candidate based on its favorable potency profile against multiple HCV genotypes 1–6 and key mutants (GT1b, IC50 = 0.2–0.4 nM; replicon GT1b, EC50 = 1.6 nM). Danoprevir has been evaluated in an open-label, single arm clinical trial in combination with ritonavir for treating COVID-19 and favourably compared to lopinavir/ritonavir in a second trial.
Sofosbuvir/velpatasvir, sold under the brand name Epclusa among others, is a fixed-dose combination medication for the treatment of hepatitis C in adults. It combines sofosbuvir and velpatasvir. It is more than 90% effective for hepatitis C genotypes one through six. It also works for hepatitis C in those who also have cirrhosis or HIV/AIDS. It is taken by mouth.
Glecaprevir/pibrentasvir (G/P), sold under the brand names Mavyret and Maviret, is a fixed-dose combination medication used to treat hepatitis C. It contains glecaprevir and pibrentasvir. It works against all six types of hepatitis C. At twelve weeks following treatment between 81% and 100% of people have no evidence of hepatitis C. It is taken once a day by mouth with food.















