
Glycolysis is the metabolic pathway that converts glucose into pyruvate. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.

An aerobic organism or aerobe is an organism that can survive and grow in an oxygenated environment. In contrast, an anaerobic organism (anaerobe) is any organism that does not require oxygen for growth. Some anaerobes react negatively or even die if oxygen is present. The ability to exhibit aerobic respiration may yield benefits to the aerobic organism, as aerobic respiration yields more energy than anaerobic respiration. In July 2020, marine biologists reported that aerobic microorganisms (mainly), in "quasi-suspended animation", were found in organically-poor sediments, up to 101.5 million years old, 250 feet below the seafloor in the South Pacific Gyre (SPG), and could be the longest-living life forms ever found.
An anaerobic organism or anaerobe is any organism that does not require molecular oxygen for growth. It may react negatively or even die if free oxygen is present. In contrast, an aerobic organism (aerobe) is an organism that requires an oxygenated environment. Anaerobes may be unicellular or multicellular. Most fungi are obligate aerobes, requiring oxygen to survive. However, some species, such as the Chytridiomycota that reside in the rumen of cattle, are obligate anaerobes; for these species, anaerobic respiration is used because oxygen will disrupt their metabolism or kill them. Deep waters of the ocean are a common anoxic environment.

Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be described as a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products.
Digestion is the breakdown of carbohydrates to yield an energy-rich compound called ATP. The production of ATP is achieved through the oxidation of glucose molecules. In oxidation, the electrons are stripped from a glucose molecule to reduce NAD+ and FAD. NAD+ and FAD possess a high energy potential to drive the production of ATP in the electron transport chain. ATP production occurs in the mitochondria of the cell. There are two methods of producing ATP: aerobic and anaerobic. In aerobic respiration, oxygen is required. Using oxygen increases ATP production from 4 ATP molecules to about 30 ATP molecules. In anaerobic respiration, oxygen is not required. When oxygen is absent, the generation of ATP continues through fermentation. There are two types of fermentation: alcohol fermentation and lactic acid fermentation.
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.

Bacteriological water analysis is a method of analysing water to estimate the numbers of bacteria present and, if needed, to find out what sort of bacteria they are. It represents one aspect of water quality. It is a microbiological analytical procedure which uses samples of water and from these samples determines the concentration of bacteria. It is then possible to draw inferences about the suitability of the water for use from these concentrations. This process is used, for example, to routinely confirm that water is safe for human consumption or that bathing and recreational waters are safe to use.

Obligate anaerobes are microorganisms killed by normal atmospheric concentrations of oxygen (20.95% O2). Oxygen tolerance varies between species, with some species capable of surviving in up to 8% oxygen, while others lose viability in environments with an oxygen concentration greater than 0.5%.
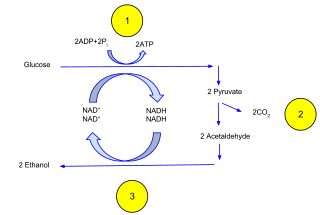
Ethanol fermentation, also called alcoholic fermentation, is a biological process which converts sugars such as glucose, fructose, and sucrose into cellular energy, producing ethanol and carbon dioxide as by-products. Because yeasts perform this conversion in the absence of oxygen, alcoholic fermentation is considered an anaerobic process. It also takes place in some species of fish where it provides energy when oxygen is scarce.

Eosin methylene blue is a selective stain for Gram-negative bacteria. EMB contains dyes that are toxic to Gram-positive bacteria. EMB is the selective and differential medium for coliforms. It is a blend of two stains, eosin and methylene blue in the ratio of 6:1. EMB is a differential microbiological medium, which slightly inhibits the growth of Gram-positive bacteria and provides a color indicator distinguishing between organisms that ferment lactose and those that do not. Organisms that ferment lactose display "nucleated colonies"—colonies with dark centers.

MacConkey agar is a selective and differential culture medium for bacteria. It is designed to selectively isolate Gram-negative and enteric bacteria and differentiate them based on lactose fermentation. Lactose fermenters turn red or pink on MacConkey agar, and nonfermenters do not change color. The media inhibits growth of Gram-positive organisms with crystal violet and bile salts, allowing for the selection and isolation of gram-negative bacteria. The media detects lactose fermentation by enteric bacteria with the pH indicator neutral red.

Methyl red, also called C.I. Acid Red 2, is an indicator dye that turns red in acidic solutions. It is an azo dye, and is a dark red crystalline powder. Methyl red is a pH indicator; it is red in pH under 4.4, yellow in pH over 6.2, and orange in between, with a pKa of 5.1. Murexide and methyl red are investigated as promising enhancers of sonochemical destruction of chlorinated hydrocarbon pollutants. Methyl red is classed by the IARC in group 3 - unclassified as to carcinogenic potential in humans.
Industrial fermentation is the intentional use of fermentation in manufacturing products useful to humans. In addition to the mass production of fermented foods and drinks, industrial fermentation has widespread applications in chemical industry. Commodity chemicals, such as acetic acid, citric acid, and ethanol are made by fermentation. Moreover, nearly all commercially produced industrial enzymes, such as lipase, invertase and rennet, are made by fermentation with genetically modified microbes. In some cases, production of biomass itself is the objective, as is the case for single-cell proteins, baker's yeast, and starter cultures for lactic acid bacteria used in cheesemaking.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.

Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food production, it may more broadly refer to any process in which the activity of microorganisms brings about a desirable change to a foodstuff or beverage. The science of fermentation is known as zymology.
Leuconostoc mesenteroides is a species of lactic acid bacteria associated with fermentation, under conditions of salinity and low temperatures. In some cases of vegetable and food storage, it was associated with pathogenicity. L. mesenteroides is approximately 0.5-0.7 µm in diameter and has a length of 0.7-1.2 µm, producing small grayish colonies that are typically less than 1.0 mm in diameter. It is facultatively anaerobic, Gram-positive, non-motile, non-sporogenous, and spherical. It often forms lenticular coccoid cells in pairs and chains, however, it can occasionally form short rods with rounded ends in long chains, as its shape can differ depending on what media the species is grown on. L. mesenteroides grows best at 30°C, but can survive in temperatures ranging from 10°C to 30°C. Its optimum pH is 5.5, but can still show growth in pH of 4.5-7.0.
The Pasteur effect describes how available oxygen inhibits ethanol fermentation, driving yeast to switch toward aerobic respiration for increased generation of the energy carrier adenosine triphosphate (ATP).

The Triple Sugar Iron (TSI) test is a microbiological test roughly named for its ability to test a microorganism's ability to ferment sugars and to produce hydrogen sulfide. It is often used to differentiate enteric bacteria including Salmonella and Shigella.

The IMViC tests are a group of individual tests used in microbiology lab testing to identify an organism in the coliform group. A coliform is a gram negative, aerobic, or facultative anaerobic rod, which produces gas from lactose within 48 hours. The presence of some coliforms indicate fecal contamination.
Glucose phosphate broth is used to perform Methyl Red (MR) test and Voges Proskauer (VP) test.










