
HU-210 is a synthetic cannabinoid that was first synthesized in 1988 from (1R,5S)-myrtenol by a group led by Raphael Mechoulam at the Hebrew University. HU-210 is 100 to 800 times more potent than natural THC from cannabis and has an extended duration of action. HU-210 is the (–)-1,1-dimethylheptyl analog of 11-hydroxy- Δ8- tetrahydrocannabinol; in some references it is called 1,1-dimethylheptyl- 11-hydroxytetrahydrocannabinol. The abbreviation "HU" stands for Hebrew University.

JWH-307 is an analgesic drug used in scientific research, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It is somewhat selective for the CB2 subtype, with a Ki of 7.7 nM at CB1 vs 3.3 nM at CB2. It was discovered by, and named after, John W. Huffman. JWH-307 was detected as an ingredient in synthetic cannabis smoking blends in 2012, initially in Germany.

JWH-250 or (1-pentyl-3-(2-methoxyphenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist at both the CB1 and CB2 receptors, with a Ki of 11 nM at CB1 and 33 nM at CB2. Unlike many of the older JWH series compounds, this compound does not have a naphthalene ring, instead occupying this position with a 2'-methoxy-phenylacetyl group, making JWH-250 a representative member of a new class of cannabinoid ligands. Other 2'-substituted analogues such as the methyl, chloro and bromo compounds are also active and somewhat more potent.

JWH-203 (1-pentyl-3-(2-chlorophenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist with approximately equal affinity at both the CB1 and CB2 receptors, having a Ki of 8.0 nM at CB1 and 7.0 nM at CB2. It was originally discovered by, and named after, Dr. John W. Huffman, but has subsequently been sold without his permission as an ingredient of synthetic cannabis smoking blends. Similar to the related 2'-methoxy compound JWH-250, the 2'-bromo compound JWH-249, and the 2'-methyl compound JWH-251, JWH-203 has a phenylacetyl group in place of the naphthoyl ring used in most aminoalkylindole cannabinoid compounds, and has the strongest in vitro binding affinity for the cannabinoid receptors of any compound in the phenylacetyl group.

Cannabicyclohexanol is a cannabinoid receptor agonist drug, developed by Pfizer in 1979. On 19 January 2009, the University of Freiburg in Germany announced that an analog of CP 47,497 was the main active ingredient in the herbal incense product Spice, specifically the 1,1-dimethyloctyl homologue of CP 47,497, which is now known as cannabicyclohexanol. The 1,1-dimethyloctyl homologue of CP 47,497 is in fact several times more potent than the parent compound, which is somewhat unexpected as the 1,1-dimethylheptyl is the most potent substituent in classical cannabinoid compounds such as HU-210.

HU-243 (AM-4056) is a synthetic cannabinoid drug that is a single enantiomer of the hydrogenated derivative of the commonly used reference agonist HU-210. It is a methylene homologue of canbisol. It is a potent agonist at both the CB1 and CB2 receptors, with a binding affinity of 0.041 nM at the CB1 receptor, making it marginally more potent than HU-210, which had an affinity of 0.061 nM in the same assay.

RCS-8 (also known as 1-(2-cyclohexylethyl)-3-(2-methoxyphenylacetyl)indole, SR-18, and BTM-8) is a synthetic cannabinoid that has been found as an ingredient of "herbal" synthetic cannabis blends. It can be described as an analogue of JWH-250 with the 1-pentyl group replaced by 1-(2-cyclohexylethyl), and can be expected to be less potent than JWH-250 (cf. JWH-007 and its cyclohexylethyl analogue). Despite not having been reported in the scientific or patent literature as yet, reputed recreational use of RCS-8 in the United States has led to it being specifically listed in a proposed 2011 amendment to the Controlled Substances Act, aiming to add a number of synthetic drugs into Schedule I. In addition, all CB1 receptor agonists of the 3-phenylacetylindole class such as RCS-8 are Schedule I Controlled Substances.
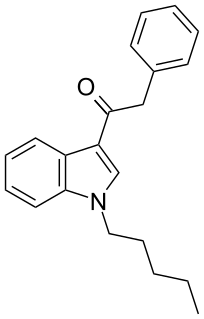
JWH-167 (1-pentyl-3-(phenylacetyl)indole) is a synthetic cannabinoid from the phenylacetylindole family, which acts as a cannabinoid agonist with about 1.75 times selectivity for CB1 with a Ki of 90 nM ± 17 and 159 nM ± 14 at CB2. Similar to the related 2'-methoxy compound JWH-250, and the 2'-chloro compound JWH-203, JWH-167 has a phenylacetyl group in place of the naphthoyl ring used in most aminoalkylindole cannabinoid compounds.
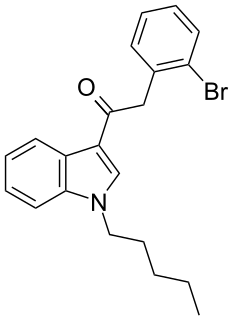
JWH-249 (1-pentyl-3-(2-bromophenylacetyl)indole) is a synthetic cannabinoid from the phenylacetylindole family, which acts as a cannabinoid agonist with about 2.4 times selectivity for CB1 with a Ki of 8.4 ± 1.8 nM and 20 ± 2 nM at CB2. Similar to the related 2'-methoxy compound JWH-250, the 2'-chloro compound JWH-203, and the 2'-methyl compound JWH-251, JWH-249 has a phenylacetyl group in place of the naphthoyl ring used in most aminoalkylindole cannabinoid compounds.
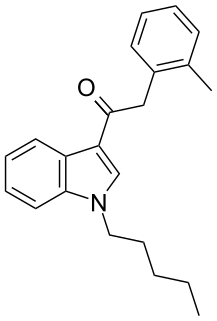
JWH-251 (1-pentyl-3-(2-methylphenylacetyl)indole) is a synthetic cannabinoid from the phenylacetylindole family, which acts as a cannabinoid agonist with about five times selectivity for CB1 with a Ki of 29 nM and 146 nM at CB2. Similar to the related 2'-methoxy compound JWH-250, the 2'-chloro compound JWH-203, and the 2'-bromo compound JWH-249, JWH-251 has a phenylacetyl group in place of the naphthoyl ring used in most aminoalkylindole cannabinoid compounds.

JWH-302 (1-pentyl-3-(3-methoxyphenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family, which acts as a cannabinoid agonist with moderate affinity at both the CB1 and CB2 receptors. It is a positional isomer of the more common drug JWH-250, though it is slightly less potent with a Ki of 17 nM at CB1, compared to 11 nM for JWH-250. Because of their identical molecular weight and similar fragmentation patterns, JWH-302 and JWH-250 can be very difficult to distinguish by GC-MS testing.

Cannabipiperidiethanone (CPE or 1-(N-methylpiperidin-2-ylmethyl)-3-(2-methoxyphenylacetyl)indole) is a synthetic cannabinoid that has been found as an ingredient of "herbal" synthetic cannabis blends sold in Japan, alongside JWH-122 and JWH-081. Its binding affinity was measured at the CB1 and CB2 receptors and it was found to have an IC50 of 591 nM at CB1 and 968 nM at CB2, making it 2.3 times and 9.4 times weaker than JWH-250 at these two targets respectively.
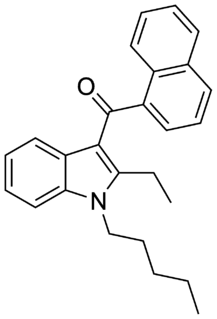
JWH-116 is a synthetic cannabinoid receptor ligand from the naphthoylindole family. It is the indole 2-ethyl derivative of related compound JWH-018. The binding affinity of JWH-116 for the CB1 receptor is reported as Ki = 52 ± 5 nM.
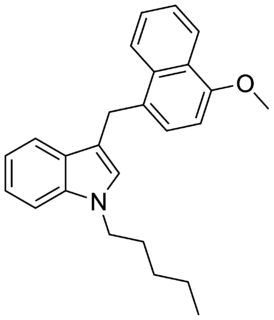
JWH-185 is a synthetic cannabinoid receptor ligand from the naphthoylindole family. It is the carbonyl-reduced derivative of related compound JWH-081. The binding affinity of JWH-185 for the CB1 receptor is reported as Ki = 17 ± 3 nM.

MAM-2201 is a drug that presumably acts as a potent agonist for the cannabinoid receptors. It had never previously been reported in the scientific or patent literature, and was first identified by laboratories in the Netherlands and Germany in June 2011 as an ingredient in synthetic cannabis smoking blends. Like RCS-4 and AB-001, MAM-2201 thus appears to be a novel compound invented by "research chemical" suppliers specifically for grey-market recreational use. Structurally, MAM-2201 is a hybrid of two known cannabinoid compounds JWH-122 and AM-2201, both of which had previously been used as active ingredients in synthetic cannabis blends before being banned in many countries.
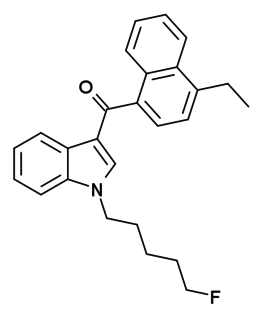
EAM-2201 is a drug that presumably acts as a potent agonist for the cannabinoid receptors. It had never previously been reported in the scientific or patent literature, and was first identified by laboratories in Japan in July 2012 as an ingredient in synthetic cannabis smoking blends Like the closely related MAM-2201 which had been first reported around a year earlier, EAM-2201 thus appears to be another novel compound invented by designer drug suppliers specifically for recreational use. Structurally, EAM-2201 is a hybrid of two known cannabinoid compounds JWH-210 and AM-2201, both of which had previously been used as active ingredients in synthetic cannabis blends before being banned in many countries.

5F-PB-22 is a designer drug which acts as a cannabinoid agonist. The structure of 5F-PB-22 appears to have been designed with an understanding of structure–activity relationships within the indole class of cannabinoids.

NE-CHMIMO (CHM-018) is an indole-based synthetic cannabinoid that is presumed to be a potent agonist of the CB1 receptor and has been sold online as a designer drug. NE-CHMIMO is the 1-cyclohexylmethyl (instead of 1-pentyl) analogue of the first-generation synthetic cannabinoid JWH-018. The corresponding cyclohexylmethyl derivative of JWH-081 had also been reported several months earlier.

MMB-CHMICA (AMB-CHMICA) is a designer drug and synthetic cannabinoid. In 2018, it was the sixth-most common synthetic cannabinoid identified in drugs seized by the Drug Enforcement Agency.




















