Isoquinoline is an individual chemical specimen - a heterocyclic aromatic organic compound - as well as the name of a family of many thousands of natural plant alkaloids, any one of which might be referred to as "an isoquinoline". It is a structural isomer of quinoline. Isoquinoline and quinoline are benzopyridines, which are composed of a benzene ring fused to a pyridine ring. In a broader sense, the term isoquinoline is used to make reference to isoquinoline derivatives. 1-Benzylisoquinoline is the structural backbone in many naturally occurring alkaloids such as papaverine. The isoquinoline ring in these natural compound derives from the aromatic amino acid tyrosine.
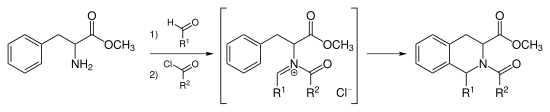
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after Carl Mannich.
In retrosynthetic analysis, a synthon is a hypothetical unit within a target molecule that represents a potential starting reagent in the retroactive synthesis of that target molecule. The term was coined in 1967 by E. J. Corey. He noted in 1988 that the "word synthon has now come to be used to mean synthetic building block rather than retrosynthetic fragmentation structures". It was noted in 1998 that the phrase did not feature very prominently in Corey's 1981 book The Logic of Chemical Synthesis, as it was not included in the index. Because synthons are charged, when placed into a synthesis an uncharged form is found commercially instead of forming and using the potentially very unstable charged synthons.
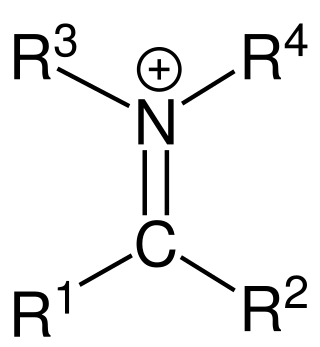
In organic chemistry, an iminium cation is a polyatomic ion with the general structure [R1R2C=NR3R4]+. They are common in synthetic chemistry and biology.
The Bischler–Napieralski reaction is an intramolecular electrophilic aromatic substitution reaction that allows for the cyclization of β-arylethylamides or β-arylethylcarbamates. It was first discovered in 1893 by August Bischler and Bernard Napieralski, in affiliation with Basle Chemical Works and the University of Zurich. The reaction is most notably used in the synthesis of dihydroisoquinolines, which can be subsequently oxidized to isoquinolines.

The Biginelli reaction is a multiple-component chemical reaction that creates 3,4-dihydropyrimidin-2(1H)-ones 4 from ethyl acetoacetate 1, an aryl aldehyde, and urea 3. It is named for the Italian chemist Pietro Biginelli.

The Petasis reaction is the multi-component reaction of an amine, a carbonyl, and a vinyl- or aryl-boronic acid to form substituted amines.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.

In organic chemistry, organocatalysis is a form of catalysis in which the rate of a chemical reaction is increased by an organic catalyst. This "organocatalyst" consists of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds. Because of their similarity in composition and description, they are often mistaken as a misnomer for enzymes due to their comparable effects on reaction rates and forms of catalysis involved.

Zincke aldehydes, or 5-aminopenta-2,4-dienals, are the product of the reaction of a pyridinium salt with two equivalents of any secondary amine, followed by basic hydrolysis. Using secondary amines the Zincke reaction takes on a different shape forming Zincke aldehydes in which the pyridine ring is ring-opened with the terminal iminium group hydrolyzed to an aldehyde. The use of the dinitrophenyl group for pyridine activation was first reported by Theodor Zincke. The use of cyanogen bromide for pyridine activation was independently reported by W. König:

Spirotryprostatin B is an indolic alkaloid found in the Aspergillus fumigatus fungus that belongs to a class of naturally occurring 2,5-diketopiperazines. Spirotryprostatin B and several other indolic alkaloids have been found to have anti-mitotic properties, and as such they have become of great interest as anti-cancer drugs. Because of this, the total syntheses of these compounds is a major pursuit of organic chemists, and a number of different syntheses have been published in the chemical literature.
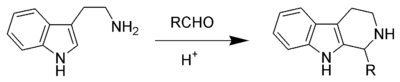
Strictosidine synthase (EC 4.3.3.2) is an enzyme in alkaloid biosynthesis that catalyses the condensation of tryptamine with secologanin to form strictosidine in a formal Pictet–Spengler reaction:

Morten Peter Meldal is a Danish chemist and Nobel laureate. He is a professor of chemistry at the University of Copenhagen in Copenhagen, Denmark. He is best known for developing the CuAAC-click reaction, concurrently with but independent of Valery V. Fokin and K. Barry Sharpless.

Indole is an aromatic, heterocyclic, organic compound with the formula C8H7N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin.

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field.
The imine Diels–Alder reaction involves the transformation of all-carbon dienes and imine dienophiles into tetrahydropyridines.
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic nitration, aromatic halogenation, aromatic sulfonation, alkylation and acylation Friedel–Crafts reaction.

Hydrogen-bond catalysis is a type of organocatalysis that relies on use of hydrogen bonding interactions to accelerate and control organic reactions. In biological systems, hydrogen bonding plays a key role in many enzymatic reactions, both in orienting the substrate molecules and lowering barriers to reaction. However, chemists have only recently attempted to harness the power of using hydrogen bonds to perform catalysis, and the field is relatively undeveloped compared to research in Lewis acid catalysis.
Rearrangements, especially those that can participate in cascade reactions, such as the aza-Cope rearrangements, are of high practical as well as conceptual importance in organic chemistry, due to their ability to quickly build structural complexity out of simple starting materials. The aza-Cope rearrangements are examples of heteroatom versions of the Cope rearrangement, which is a [3,3]-sigmatropic rearrangement that shifts single and double bonds between two allylic components. In accordance with the Woodward-Hoffman rules, thermal aza-Cope rearrangements proceed suprafacially. Aza-Cope rearrangements are generally classified by the position of the nitrogen in the molecule :
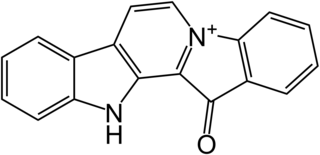
Fascaplysin is a marine alkaloid based on 12H-pyrido[1–2-a:3,4-b′]diindole ring system. It was first isolated as a red pigment from the marine sponge Fascaplysinopsis bergquist collected in the South Pacific near Fiji in 1988. Fascaplysin possesses a broad range of in vitro biological activities including analgesic, antimicrobial, antifungal, antiviral, antimalarial, anti-angiogenic, and antiproliferative activity against numerous cancer cell lines.