Pyrimidine is an aromatic heterocyclic organic compound similar to pyridine. One of the three diazines, it has the nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine and pyridazine. In nucleic acids, three types of nucleobases are pyrimidine derivatives: cytosine (C), thymine (T), and uracil (U).
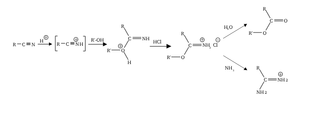
The Pinner reaction refers to the acid catalysed reaction of a nitrile with an alcohol to form an imino ester salt ; this is sometimes referred to as a Pinner salt. The reaction is named after Adolf Pinner, who first described it in 1877. Pinner salts are themselves reactive and undergo additional nucleophilic additions to give various useful products:
Amidines are a class of oxoacid derivatives.

Cyanuric chloride is an organic compound with the formula (NCCl)3. This white solid is the chlorinated derivative of 1,3,5-triazine. It is the trimer of cyanogen chloride. Cyanuric chloride is the main precursor to the popular but controversial herbicide atrazine.

Hexazinone is an organic compound that is used as a broad spectrum herbicide. It is a colorless solid. It exhibits some solubility in water but is highly soluble in most organic solvents except alkanes. A member of the triazine class herbicides, it is manufactured by DuPont and sold under the trade name Velpar.
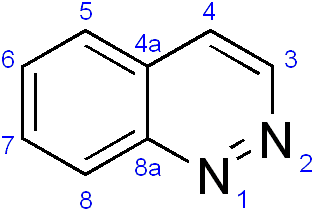
Cinnoline is an aromatic heterocyclic compound with the formula C8H6N2. It is isomeric with other naphthyridines including quinoxaline, phthalazine and quinazoline.
1,3,5-triazine, also called s-triazine, is an organic chemical compound with the formula (HCN)3. It is a six-membered heterocyclic aromatic ring, one of several isomeric triazines. S-triazine and its derivatives are useful in a variety of applications.

1,5-Diazabicyclo[4.3.0]non-5-ene (DBN) is a chemical compound with the formula C7H12N2. It is an amidine base used in organic synthesis. A related compound with related functions is 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). The relatively complex nature of the formal names for DBU and DBN (hence the common use of acronyms) reflects the fact that these compounds are bicyclic and contain several functional groups.

2,4,6-Tris(trinitromethyl)-1,3,5-triazine is a chemical compound that is a derivative of triazine first prepared in 1995. It is synthesized by destructive nitration of 2,4,6-tricarboxyl-1,3,5-triazine. It is noteworthy for having more nitro groups than it does carbon atoms, so could be used as an oxygen source, or added to oxygen-poor explosives to increase their power.
The Bamberger triazine synthesis in organic chemistry is a classic organic synthesis of a triazine first reported by Eugen Bamberger in 1892.

Simazine is an herbicide of the triazine class. The compound is used to control broad-leaved weeds and annual grasses.
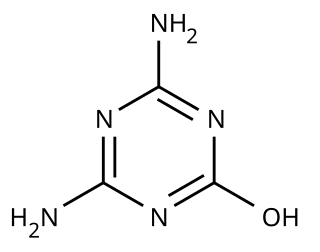
Ammeline (4,6-diamino-2-hydroxy-1,3,5-triazine) is a triazine derivative. It is the hydrolysis product of melamine.
In enzymology, a hydroxydechloroatrazine ethylaminohydrolase (EC 3.5.99.3) is an enzyme that catalyzes the chemical reaction

Oxazoline is a five-membered heterocyclic chemical compound containing one atom each of oxygen and nitrogen. It was likely first synthesized in 1884 but it was not until 5 years later that Siegmund Gabriel correctly assigned the structure. It was named in-line with the Hantzsch–Widman nomenclature and is part of a family of heterocyclic compounds, where it exists between oxazole and oxazolidine in terms of saturation.
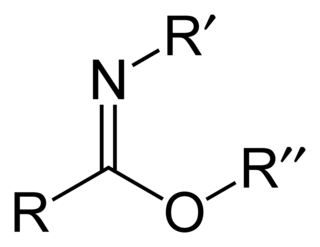
Carboximidates are organic compounds, which can be thought of as esters formed between a carboximidic acid and an alcohol, with the general formula R-C(=NR')OR".

In chemistry, hexahydro-1,3,5-triazine is a class of heterocyclic compounds with the formula (CH2NR)3. They are reduced derivatives of 1,3,5-triazine, which have the formula (CHN)3, a family of aromatic heterocycles.
The Boger pyridine synthesis is a cycloaddition approach to the formation of pyridines named after its inventor Dale L. Boger, who first reported it in 1981. The reaction is a form of inverse-electron demand Diels-Alder reaction in which an enamine reacts with a 1,2,4-triazine to form the pyridine nucleus. The reaction is especially useful for accessing pyridines that would be difficult or impossible to access via other methods and has been used in the total synthesis of several complicated natural products.

Cynazine is a herbicide that belongs to the group of triazines. Cyanazine inhibits photosynthesis and is therefore used as a herbicide.















