
A placebo is a substance or treatment which is designed to have no therapeutic value. Common placebos include inert tablets, inert injections, sham surgery, and other procedures.

Auriculotherapy is a form of alternative medicine based on the idea that the ear is a micro system and a external organ, which reflects the entire body, represented on the auricle, the outer portion of the ear. Conditions affecting the physical, mental or emotional health of the patient are assumed to be treatable by stimulation of the surface of the ear exclusively. Similar mappings are used by several modalities, including the practices of reflexology and iridology. These mappings are not based on or supported by any Western medical or scientific evidence, and are therefore considered to be pseudoscience.
A placebo is a substance or treatment without intrinsic therapeutic value, but administered as if it were a therapy, either in medical treatment or in clinical trials.
A nocebo effect is said to occur when negative expectations of the patient regarding a treatment cause the treatment to have a more negative effect than it otherwise would have. For example, when a patient anticipates a side effect of a medication, they can experience that effect even if the "medication" is actually an inert substance. The complementary concept, the placebo effect, is said to occur when positive expectations improve an outcome. The nocebo effect is also said to occur in someone who falls ill owing to the erroneous belief that they were exposed to a toxin, e.g. SARS-CoV-2 vaccine adulterants, or to a physical phenomenon they believe is harmful, such as EM radiation.
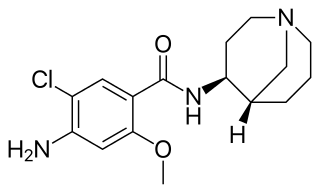
Renzapride is a prokinetic agent and antiemetic which acts as a full 5-HT4 agonist and partial 5-HT3 antagonist. It also functions as a 5-HT2B antagonist and has some affinity for the 5-HT2A and 5-HT2C receptors.

Rilmenidine is a prescription medication for the treatment of hypertension. It is marketed under the brand names Albarel, Hyperium, Iterium and Tenaxum.
Mepolizumab, sold under the brand name Nucala, is a humanized monoclonal antibody used for the treatment of severe eosinophilic asthma, eosinophilic granulomatosis, and hypereosinophilic syndrome (HES). It recognizes and blocks interleukin-5 (IL-5), a signalling protein of the immune system.

Ted Jack Kaptchuk is an American medical researcher who holds professorships in medicine and in global health and social medicine at Harvard Medical School. He researches the placebo effect within the field of placebo studies.
The following outline is provided as an overview of and topical guide to clinical research:

Placebo-controlled studies are a way of testing a medical therapy in which, in addition to a group of subjects that receives the treatment to be evaluated, a separate control group receives a sham "placebo" treatment which is specifically designed to have no real effect. Placebos are most commonly used in blinded trials, where subjects do not know whether they are receiving real or placebo treatment. Often, there is also a further "natural history" group that does not receive any treatment at all.

Irving Kirsch is an American psychologist and academic. He is the Associate Director of the Program in Placebo Studies and a lecturer in medicine at the Harvard Medical School and Beth Israel Deaconess Medical Center. He is also professor emeritus of psychology at the Universities of Hull and Plymouth in the United Kingdom, and the University of Connecticut in the United States. Kirsch is a leading researcher within the field of placebo studies who is noted for his work on placebo effects, antidepressants, expectancy, and hypnosis. He is the originator of response expectancy theory, and his analyses of clinical trials of antidepressants have influenced official treatment guidelines in the United Kingdom. He is the author of the 2009 book The Emperor's New Drugs, which argued most antidepressant medication is effective primarily due to placebo effects.
Olokizumab is an immunomodulator. It binds to interleukin 6. Hence acting as an Anti-IL-6 therapeutic aimed at inflammatory disease e.g. rheumatoid arthritis (RA).

Brexpiprazole, sold under the brand name Rexulti among others, is a medication used for the treatment of major depressive disorder, schizophrenia, and agitation associated with dementia due to Alzheimer's disease. It is an atypical antipsychotic.
Eldelumab is a fully human monoclonal antibody that targets chemokine ligand 10 (CXCL10)/Interferon-γ-inducible protein-10 (IP-10) designed for the treatment of Crohn's disease and ulcerative colitis.

The Emperor's New Drugs – Exploding the Antidepressant Myth is a 2009 book by Irving Kirsch, arguing that the chemical imbalance theory of depression is wrong and that antidepressants have little or no direct effect on depression but, because of their common or serious side-effects, they are powerful active placebos.
Anifrolumab, sold under the brand name Saphnelo, is a monoclonal antibody used for the treatment of systemic lupus erythematosus (SLE). It binds to the type I interferon receptor, blocking the activity of type I interferons such as interferon-α and interferon-β.
The philosophy of medicine is a branch of philosophy that explores issues in theory, research, and practice within the field of health sciences. More specifically in topics of epistemology, metaphysics, and medical ethics, which overlaps with bioethics. Philosophy and medicine, both beginning with the ancient Greeks, have had a long history of overlapping ideas. It was not until the nineteenth century that the professionalization of the philosophy of medicine came to be. In the late twentieth century, debates among philosophers and physicians ensued of whether the philosophy of medicine should be considered a field of its own from either philosophy or medicine. A consensus has since been reached that it is in fact a distinct discipline with its set of separate problems and questions. In recent years there have been a variety of university courses, journals, books, textbooks and conferences dedicated to the philosophy of medicine.

Filgotinib, sold under the brand name Jyseleca, is a medication used for the treatment of rheumatoid arthritis (RA). It was developed by the Belgian-Dutch biotech company Galapagos NV.
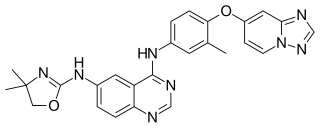
Tucatinib, sold under the brand name Tukysa, is an anticancer medication used for the treatment of HER2-positive breast cancer. It is a small molecule inhibitor of HER2. It was developed by Array BioPharma and licensed to Cascadian Therapeutics.

Zuranolone, sold under the brand name Zurzuvae, is a medication used for the treatment of postpartum depression. It is taken by mouth.










