In organic chemistry, a sulfide or thioether is an organosulfur functional group with the connectivity R−S−R' as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application.

Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene. Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents.

In organic chemistry, an allyl group is a substituent with the structural formula −CH2−HC=CH2. It consists of a methylene bridge attached to a vinyl group. The name is derived from the scientific name for garlic, Allium sativum. In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "Schwefelallyl". The term allyl applies to many compounds related to H2C=CH−CH2, some of which are of practical or of everyday importance, for example, allyl chloride.
In organic chemistry a halohydrin is a functional group in which a halogen and a hydroxyl are bonded to adjacent carbon atoms, which otherwise bear only hydrogen or hydrocarbyl groups. The term only applies to saturated motifs, as such compounds like 2-chlorophenol would not normally be considered halohydrins. Megatons of some chlorohydrins, e.g. propylene chlorohydrin, are produced annually as precursors to polymers.
The Pummerer rearrangement is an organic reaction whereby an alkyl sulfoxide rearranges to an α-acyloxy–thioether (monothioacetal-ester) in the presence of acetic anhydride.
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.

In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.

In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent.

Sodium sulfide is a chemical compound with the formula Na2S, or more commonly its hydrate Na2S·9H2O. Both the anhydrous and the hydrated salts in pure crystalline form are colorless solids, although technical grades of sodium sulfide are generally yellow to brick red owing to the presence of polysulfides and commonly supplied as a crystalline mass, in flake form, or as a fused solid. They are water-soluble, giving strongly alkaline solutions. When exposed to moist air, Na2S and its hydrates emit hydrogen sulfide, an extremely toxic, flammable and corrosive gas which smells like rotten eggs.
In organic chemistry, a cyclophane is a hydrocarbon consisting of an aromatic unit and a chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known. Cyclophanes are well-studied examples of strained organic compounds.
Organoselenium chemistry is the science exploring the properties and reactivity of organoselenium compounds, chemical compounds containing carbon-to-selenium chemical bonds. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.

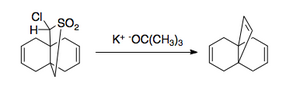
The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid, but usually either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds.

2,3-Sigmatropic rearrangements are a type of sigmatropic rearrangements and can be classified into two types. Rearrangements of allylic sulfoxides, amine oxides, selenoxides are neutral. Rearrangements of carbanions of allyl ethers are anionic. The general scheme for this kind of rearrangement is:
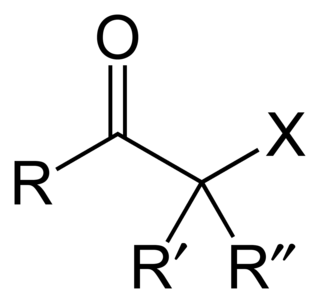
In organic chemistry, an α-halo ketone is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. α-Halo ketones are alkylating agents. Prominent α-halo ketones include phenacyl bromide and chloroacetone.
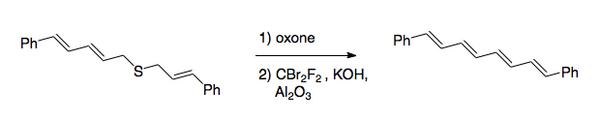
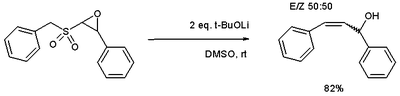
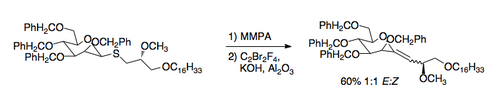
The Julia olefination (also known as the Julia–Lythgoe olefination) is the chemical reaction used in organic chemistry of phenyl sulfones (1) with aldehydes (or ketones) to give alkenes (olefins)(3) after alcohol functionalization and reductive elimination using sodium amalgam or SmI2. The reaction is named after the French chemist Marc Julia.
In inorganic chemistry, sulfonyl halide groups occur when a sulfonyl functional group is singly bonded to a halogen atom. They have the general formula RSO2X, where X is a halogen. The stability of sulfonyl halides decreases in the order fluorides > chlorides > bromides > iodides, all four types being well known. The sulfonyl chlorides and fluorides are of dominant importance in this series.
In organic chemistry, a homologation reaction, also known as homologization, is any chemical reaction that converts the reactant into the next member of the homologous series. A homologous series is a group of compounds that differ by a constant unit, generally a methylene group. The reactants undergo a homologation when the number of a repeated structural unit in the molecules is increased. The most common homologation reactions increase the number of methylene units in saturated chain within the molecule. For example, the reaction of aldehydes or ketones with diazomethane or methoxymethylenetriphenylphosphine to give the next homologue in the series.
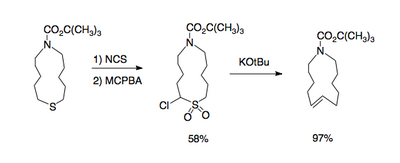
Desulfonylation reactions are chemical reactions leading to the removal of a sulfonyl group from organic compounds. As the sulfonyl functional group is electron-withdrawing, methods for cleaving the sulfur–carbon bonds of sulfones are typically reductive in nature. Olefination or replacement with hydrogen may be accomplished using reductive desulfonylation methods.
An insertion reaction is a chemical reaction where one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.: