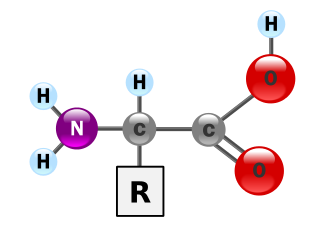
Alanine (symbol Ala or A) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated form, −NH3+, under biological conditions), an α-carboxylic acid group (which is in the deprotonated form, −COO−, under biological conditions), and a side chain methyl group, making it a nonpolar, aliphatic amino acid. It is non-essential in humans: because the body can synthesize it, it does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG).
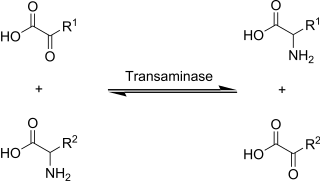
Transamination, a chemical reaction that transfers an amino group to a ketoacid to form new amino acids. This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential amino acids to nonessential amino acids.

A cyanohydrin is a functional group found in organic compounds in which a cyano and a hydroxy group are attached to the same carbon atom. The general formula is R2C(OH)CN, where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids. Cyanohydrins can be formed by the cyanohydrin reaction, which involves treating a ketone or an aldehyde with hydrogen cyanide (HCN) in the presence of excess amounts of sodium cyanide (NaCN) as a catalyst:
Edman degradation, developed by Pehr Edman, is a method of sequencing amino acids in a peptide. In this method, the amino-terminal residue is labeled and cleaved from the peptide without disrupting the peptide bonds between other amino acid residues.
Aspartate transaminase (AST) or aspartate aminotransferase, also known as AspAT/ASAT/AAT or (serum) glutamic oxaloacetic transaminase, is a pyridoxal phosphate (PLP)-dependent transaminase enzyme that was first described by Arthur Karmen and colleagues in 1954. AST catalyzes the reversible transfer of an α-amino group between aspartate and glutamate and, as such, is an important enzyme in amino acid metabolism. AST is found in the liver, heart, skeletal muscle, kidneys, brain, and red blood cells. Serum AST level, serum ALT level, and their ratio are commonly measured clinically as biomarkers for liver health. The tests are part of blood panels.

β-peptides consist of β amino acids, which have their amino group bonded to the β carbon rather than the α carbon as in the 20 standard biological amino acids. The only common naturally occurring β amino acid is β-alanine; although it is used as a component of larger bioactive molecules, β-peptides in general do not appear in nature. For this reason β-peptide-based antibiotics are being explored as ways of evading antibiotic resistance. Early studies in this field were published in 1996 by the group of Dieter Seebach and that of Samuel Gellman.
In chemistry, a multi-component reaction, sometimes referred to as a "Multi-component Assembly Process", is a chemical reaction where three or more compounds react to form a single product. By definition, multicomponent reactions are those reactions whereby more than two reactants combine in a sequential manner to give highly selective products that retain majority of the atoms of the starting material.
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, was discovered by German chemist Adolph Strecker, and is a term used for a series of chemical reactions that synthesize an amino acid from an aldehyde or ketone. The aldehyde is condensed with ammonium chloride in the presence of potassium cyanide to form an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino acid. In the original Strecker reaction acetaldehyde, ammonia, and hydrogen cyanide combined to form after hydrolysis alanine.
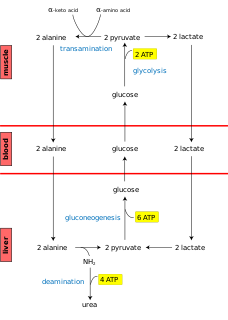
The Cahill cycle, also known as the alanine cycle or glucose-alanine cycle, is the series of reactions in which amino groups and carbons from muscle are transported to the liver. It is quite similar to the Cori cycle in the cycling of nutrients between skeletal muscle and the liver. When muscles degrade amino acids for energy needs, the resulting nitrogen is transaminated to pyruvate to form alanine. This is performed by the enzyme alanine transaminase (ALT), which converts L-glutamate and pyruvate into α-ketoglutarate and L-alanine. The resulting L-alanine is shuttled to the liver where the nitrogen enters the urea cycle and the pyruvate is used to make glucose.

Deamidation is a chemical reaction in which an amide functional group in the side chain of the amino acids asparagine or glutamine is removed or converted to another functional group. Typically, asparagine is converted to aspartic acid or isoaspartic acid. Glutamine is converted to glutamic acid or pyroglutamic acid (5-oxoproline). In a protein or peptide, these reactions are important because they may alter its structure, stability or function and may lead to protein degradation. The resulting mass adduct is a +1 Da shift.
Oxidative deamination is a form of deamination that generates α-keto acids and other oxidized products from amine-containing compounds, and occurs largely in the liver and kidney. Oxidative deamination is an important step in the catabolism of amino acids, generating a more metabolizable form of the amino acid, and also generating ammonia as a toxic byproduct. The ammonia generated in this process can then be neutralized into urea via the urea cycle.
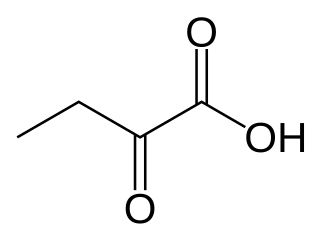
α-Ketobutyric acid is an organic compound with the formula CH3CH2C(O)CO2H. It is a colorless solid that melts just above room temperature. Its conjugate base α-ketobutyrate is the predominant form found in nature (near neutral pH). It results from the lysis of cystathionine. It is also one of the degradation products of threonine, produced by the catabolism of the amino acid by threonine dehydratase. It is also produced by the degradation of homocysteine and the metabolism of methionine.

Adolph Strecker was a German chemist who is remembered primarily for his work with amino acids.

Phenylacetaldehyde is an organic compound used in the synthesis of fragrances and polymers.
Strecker is a German surname. Notable people with the surname include:
N-Sulfinyl imines are a class of imines bearing a sulfinyl group attached to nitrogen. These imines display usefully stereoselectivity reactivity and due to the presence of the chiral electron withdrawing N-sulfinyl group. They allow 1,2-addition of organometallic reagents to imines. The N-sulfinyl group exerts powerful and predictable stereodirecting effects resulting in high levels of asymmetric induction. Racemization of the newly created carbon-nitrogen stereo center is prevented because anions are stabilized at nitrogen. The sulfinyl chiral auxiliary is readily removed by simple acid hydrolysis. The addition of organometallic reagents to N-sulfinyl imines is the most reliable and versatile method for the asymmetric synthesis of amine derivatives. These building blocks have been employed in the asymmetric synthesis of numerous biologically active compounds.

Methional is an organic compound with the formula CH3SCH2CH2CHO. It is a colorless liquid that is a degradation product of methionine. It is a notable flavor in potato-based snacks, namely potato chips, one of the most popular foods containing methional. Traces of the compound can also be found in black tea and green tea based products. Methional contains both aldehyde and thioether functional groups. It is readily soluble in alcohol solvents, including propylene glycol and dipropylene glycol.
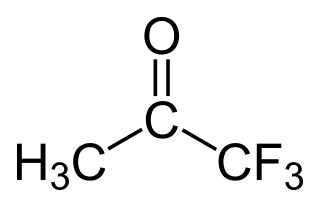
Trifluoroacetone (1,1,1-trifluoroacetone) is an organofluorine compound with the chemical formula CF3C(O)CH3. The compound is a colorless liquid with chloroform-like odour.

Phenylglycine is the organic compound with the formula C6H5CH(NH2)CO2H. It is a non-proteinogenic alpha amino acid related to alanine, but with a phenyl group in place of methyl. It is a white solid. The compound exhibits some biological activity.