Ductility is a measure of a material's ability to undergo significant plastic deformation before rupture, which may be expressed as percent elongation or percent area reduction from a tensile test. According to Shigley's Mechanical Engineering Design significant denotes about 5.0 percent elongation. See also Eq. 2–12, p. 50 for definitions of percent elongation and percent area reduction. Ductility is often characterized by a material's ability to be stretched into a wire.

Martensite is named after the German metallurgist Adolf Martens (1850–1914). The term most commonly refers to a very hard form of steel crystalline structure, but can also refer to any crystal structure that is formed by diffusionless transformation. Martensite includes a class of hard minerals that occur as lath- or plate-shaped crystal grains.

Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron, with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 K (727 °C); other alloys of steel have different eutectoid temperatures. The austenite allotrope is named after Sir William Chandler Roberts-Austen (1843–1902); it exists at room temperature in stainless steel.
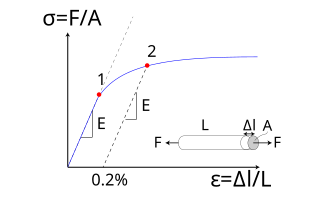
The relationship between the stress and strain that a particular material displays is known as that particular material's stress–strain curve. It is unique for each material and is found by recording the amount of deformation (strain) at distinct intervals of tensile or compressive loading (stress). These curves reveal many of the properties of a material.

Bainite is a plate-like microstructure that forms in steels at temperatures of 125–550 °C. First described by E. S. Davenport and Edgar Bain, it is one of the products that may form when austenite is cooled past a temperature where it no longer is thermodynamically stable with respect to ferrite, cementite, or ferrite and cementite. Davenport and Bain originally described the microstructure as being similar in appearance to tempered martensite.

Carbon steel is a steel with carbon content up to 2.1% by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:
Magnetic shape memory alloys (MSMAs), also called ferromagnetic shape memory alloys (FSMA), are particular shape memory alloys which produce forces and deformations in response to a magnetic field. The thermal shape memory effect has been obtained in these materials, too.

Maraging steels are steels that are known for possessing superior strength and toughness without losing ductility. Aging refers to the extended heat-treatment process. These steels are a special class of low-carbon ultra-high-strength steels that derive their strength not from carbon, but from precipitation of intermetallic compounds. The principal alloying element is 15 to 25 wt.% nickel. Secondary alloying elements, which include cobalt, molybdenum and titanium, are added to produce intermetallic precipitates. Original development was carried out on 20 and 25 wt.% Ni steels to which small additions of Al, Ti, and Nb were made; a rise in the price of cobalt in the late 1970s led to the development of cobalt-free maraging steels.
Cryogenic hardening is a cryogenic treatment process where the material is cooled to approximately −185 °C (−301 °F), usually using liquid nitrogen. It can have a profound effect on the mechanical properties of certain steels, provided their composition and prior heat treatment are such that they retain some austenite at room temperature. It is designed to increase the amount of martensite in the steel's crystal structure, increasing its strength and hardness, sometimes at the cost of toughness. Presently this treatment is being practiced over tool steels, high-carbon, and high-chromium steels to obtain excellent wear resistance. Recent research shows that there is precipitation of fine carbides in the matrix during this treatment which imparts very high wear resistance to the steels.

A superalloy, or high-performance alloy, is an alloy that exhibits several key characteristics: excellent mechanical strength, resistance to thermal creep deformation, good surface stability, and resistance to corrosion or oxidation. The crystal structure is typically face-centered cubic austenitic. Examples of such alloys are Hastelloy, Inconel, Waspaloy, Rene alloys, Incoloy, MP98T, TMS alloys, and CMSX single crystal alloys.
Hardening is a metallurgical metalworking process used to increase the hardness of a metal. The hardness of a metal is directly proportional to the uniaxial yield stress at the location of the imposed strain. A harder metal will have a higher resistance to plastic deformation than a less hard metal.
Annealing, in metallurgy and materials science, is a heat treatment that alters the physical and sometimes chemical properties of a material to increase its ductility and reduce its hardness, making it more workable. It involves heating a material above its recrystallization temperature, maintaining a suitable temperature for a suitable amount of time, and then cooling.
Austenitic stainless steel is a specific type of stainless steel alloy. Stainless steels may be classified by their crystalline structure into four main types: austenitic, ferritic,martensitic and duplex. These stainless steels possess austenite as their primary crystalline structure. This austenite crystalline structure is achieved by sufficient additions of the austenite stabilizing elements nickel, manganese and nitrogen. Due to their crystalline structure austenitic steels are not hardenable by heat treatment and are essentially non-magnetic.

Alloy steel is steel that is alloyed with a variety of elements in total amounts between 1.0% and 50% by weight to improve its mechanical properties. Alloy steels are broken down into two groups: low alloy steels and high alloy steels. The difference between the two is somewhat arbitrary: Smith and Hashemi define the difference at 4.0%, while Degarmo, et al., define it at 8.0%. Most commonly, the phrase "alloy steel" refers to low-alloy steels.
Methods have been devised to modify the yield strength, ductility, and toughness of both crystalline and amorphous materials. These strengthening mechanisms give engineers the ability to tailor the mechanical properties of materials to suit a variety of different applications. For example, the favorable properties of steel result from interstitial incorporation of carbon into the iron lattice. Brass, a binary alloy of copper and zinc, has superior mechanical properties compared to its constituent metals due to solution strengthening. Work hardening has also been used for centuries by blacksmiths to introduce dislocations into materials, increasing their yield strengths.

Dual-phase steel (DP steel) is a high-strength steel that has a ferritic–martensitic microstructure. DP steels are produced from low or medium carbon steels that are quenched from a temperature above A1 but below A3 determined from continuous cooling transformation diagram. This results in a microstructure consisting of a soft ferrite matrix containing islands of martensite as the secondary phase (martensite increases the tensile strength). Therefore, the overall behaviour of DP steels is governed by the volume fraction, morphology (size, aspect ratio, interconnectivity, etc.), the grain size and the carbon content. For achieving these microstructures, DP steels typically contain 0.06–0.15 wt.% C and 1.5-3% Mn (the former strengthens the martensite, and the latter causes solid solution strengthening in ferrite, while both stabilize the austenite), Cr & Mo (to retard pearlite or bainite formation), Si (to promote ferrite transformation), V and Nb (for precipitation strengthening and microstructure refinement). The desire to produce high strength steels with formability greater than microalloyed steel led the development of DP steels in the 1970s.
TRIP steel are a class of high-strength steel alloys typically used in naval and marine applications and in the automotive industry. TRIP stands for "Transformation induced plasticity," which implies a phase transformation in the material, typically when a stress is applied. These alloys are known to possess an outstanding combination of strength and ductility.

Mangalloy, also called manganese steel or Hadfield steel, is an alloy steel containing an average of around 13% manganese. Mangalloy is known for its high impact strength and resistance to abrasion once in its work-hardened state.