
Dextrorphan (DXO) is a psychoactive drug of the morphinan class which acts as an antitussive or cough suppressant and in high doses a dissociative hallucinogen. It is the dextrorotatory enantiomer of racemorphan; the levorotatory enantiomer is levorphanol. Dextrorphan is produced by O-demethylation of dextromethorphan by CYP2D6. Dextrorphan is an NMDA antagonist and contributes to the psychoactive effects of dextromethorphan.

Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others. Typical examples include compounds such as morphine, codeine, and dextromethorphan (DXM). Despite related molecular structures, the pharmacological profiles and mechanisms of action between the various types of morphinan substances can vary substantially. They tend to function either as μ-opioid receptor agonists (analgesics), or NMDA receptor antagonists (dissociatives).

Methorphan comes in two isomeric forms, each with differing pharmacology and effects:

Zipeprol is a centrally acting cough suppressant developed in France in the 1970s. It is not a morphinan derivative. Zipeprol acts as a local anaesthetic and has mucolytic, antihistamine and anticholinergic properties. It is sold with several brand names such as Zinolta and Respilene. It is not available in the United States or Canada and has been discontinued in Europe. It is still available in some countries in Asia and South America.
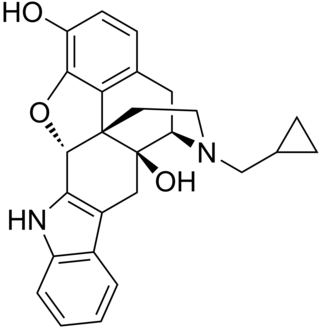
Naltrindole is a highly potent, highly selective delta opioid receptor antagonist used in biomedical research. In May 2012 a paper was published in Nature with the structure of naltrindole in complex with the mouse δ-opioid G-protein coupled receptor, solved by X-ray crystallography.

Phenomorphan is an opioid analgesic. It is not currently used in medicine, but has similar side-effects to other opiates, which include itching, nausea and respiratory depression.

Cyprodime is an opioid antagonist from the morphinan family of drugs.
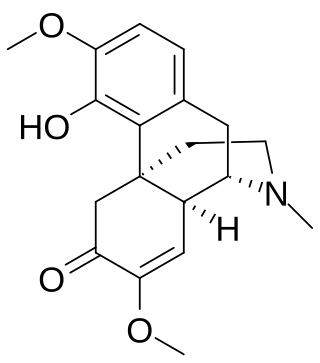
Sinomenine or cocculine is an alkaloid found in the root of the climbing plant Sinomenium acutum which is native to Japan and China. The plant is traditionally used in herbal medicine in these countries for rheumatism and arthritis. However, its analgesic action against other kinds of pain is limited. Sinomenine is a morphinan derivative, related to opioids such as levorphanol and the non-opioid cough suppressant dextromethorphan. Its anti-rheumatic effects are thought to be primarily mediated via release of histamine, but other effects such as inhibition of prostaglandin, leukotriene and nitric oxide synthesis may also be involved.
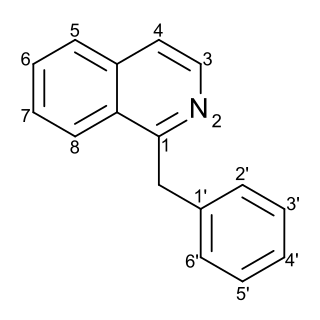
Substitution of the heterocycle isoquinoline at the C1 position by a benzyl group provides 1‑benzylisoquinoline, the most widely examined of the numerous benzylisoquinoline structural isomers. The 1-benzylisoquinoline moiety can be identified within numerous compounds of pharmaceutical interest, such as moxaverine; but most notably it is found within the structures of a wide variety of plant natural products, collectively referred to as benzylisoquinoline alkaloids. This class is exemplified in part by the following compounds: papaverine, noscapine, codeine, morphine, apomorphine, berberine, tubocurarine.

Ro4-1539 (furethylnorlevorphanol) is an opioid analgesic drug from the morphinan series that was discovered by the pharmaceutical company Hoffmann–La Roche in the 1950s. It acts as a potent μ-opioid agonist, and was found to be around 30-60 times more potent than the related drug levorphanol in animal experiments. Although it has high potency, long duration, and good therapeutic index, Ro4-1539 had no particular clinical advantages over other available opioid drugs, and was never commercially marketed.

Racemorphan, or morphanol, is the racemic mixture of the two stereoisomers of 17-methylmorphinan-3-ol, each with differing pharmacology and effects:

Cyclorphan is an opioid analgesic of the morphinan family that was never marketed. It acts as a μ-opioid receptor (MOR) weak partial agonist or antagonist, κ-opioid receptor (KOR) full agonist, and, to a much lesser extent, δ-opioid receptor (DOR) agonist. The drug was first synthesized in 1964 by scientists at Research Corporation. In clinical trials, it had relatively long duration, good absorption, and provided strong pain relief but produced psychotomimetic effects via KOR activation, so its development was not continued.

Naloxonazine is a potent, irreversible μ-opioid receptor antagonist. Naloxonazine forms spontaneously in acidic solutions of naloxazone, and may be responsible for much or all of the irreversible μ opioid receptor binding displayed by the latter.

Norlevorphanol is an opioid analgesic of the morphinan family that was never marketed. It is the levo-isomer of 3-hydroxymorphinan (morphinan-3-ol). Norlevorphanol is a Schedule I Narcotic controlled substance in the United States with an ACSCN of 9634 and in 2014 it had an annual aggregate manufacturing quota of 52 grams. It is used as the hydrobromide and hydrochloride (0.870).

Dextrallorphan (DXA) is a chemical of the morphinan class that is used in scientific research. It acts as a σ1 receptor agonist and NMDA receptor antagonist. It has no significant affinity for the σ2, μ-opioid, or δ-opioid receptor, or for the serotonin or norepinephrine transporter. As an NMDA receptor antagonist, in vivo, it is approximately twice as potent as dextromethorphan, and five-fold less potent than dextrorphan.
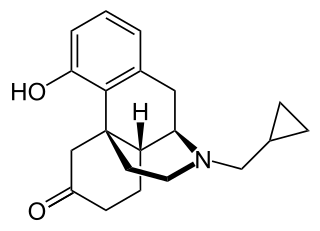
Ketorfanol, or ketorphanol, is an opioid analgesic of the morphinan family that was found to possess "potent antiwrithing activity" in animal assays but was never marketed. It is a 17-cycloalkylmethyl derivative of morphinan and as such, is closely related structurally to butorphanol, cyclorphan, oxilorphan, proxorphan, and xorphanol, which act preferentially as κ-opioid receptor agonists and to a lesser extent as μ-opioid receptor partial agonists/antagonists.

Dimethylaminopivalophenone is an opioid analgesic with a potency ½ that of morphine. It was initially discovered by Russian scientists in 1954 and subsequently rediscovered in the US in 1969. Its LD50 in mice is 83 mg/kg. It has never been marketed commercially.

Oxymorphone-3-methoxynaltrexonazine (OM-3-MNZ) is a morphinan-based opioid that acts as a selective μ-opioid receptor agonist, unlike the closely related mixed agonist-antagonist Oxymorphonenaltrexonazine.
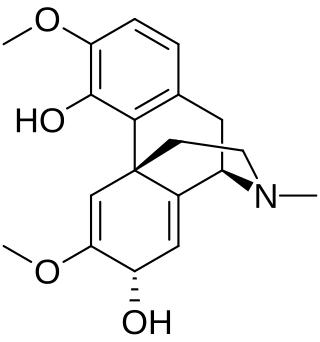
Salutaridinol is a modified benzyltetrahydroisoquinoline alkaloid with the formula C19H23NO4. It is produced in the secondary metabolism of the opium poppy Papaver somniferum (Papaveraceae) as an intermediate in the biosynthetic pathway that generates morphine. As an isoquinoline alkaloid, it is fundamentally derived from tyrosine as part of the shikimate pathway of secondary metabolism. Salutaridinol is a product of the enzyme salutaridine: NADPH 7-oxidoreductase and the substrate for the enzyme salutaridinol 7-O-acetyltransferase, which are two of the four enzymes in the morphine biosynthesis pathway that generates morphine from (R)-reticuline. Salutaridinol's unique position adjacent to two of the four enzymes in the morphine biosynthesis pathway gives it an important role in enzymatic, genetic, and synthetic biology studies of morphine biosynthesis. Salutaridinol levels are indicative of the flux through the morphine biosynthesis pathway and the efficacy of both salutaridine: NADPH 7-oxidoreductase and salutaridinol 7-O-acetyltransferase.
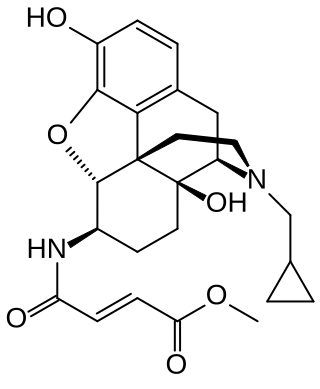
β-Funaltrexamine (β-FNA) is an irreversible opioid antagonist that was used to create the first crystal structure of the μ-opioid receptor (MOR). It is selective for antagonism of the MOR over the δ-opioid receptor (DOR) and κ-opioid receptor (KOR). Chemically, it is a naltrexone derivative with a methyl-fumaramide group in the 6-position. In addition to its MOR irreversible antagonism, β-FNA is a reversible agonist of the κ-opioid receptor (KOR) and produces KOR-mediated analgesic effects in animals. This has limited its usefulness and contributed to the development of methocinnamox as a more selective functionally irreversible antagonist of the MOR with no significant opioid agonistic actions.




















