This article needs additional citations for verification .(July 2011) (Learn how and when to remove this template message) |
The tetrad test is a series of behavioral paradigms in which rodents treated with cannabinoids such as THC show effects. [1] [2] It is widely used for screening drugs that induce cannabinoid receptor-mediated effects in rodents. The four behavioral components of the tetrad are spontaneous activity, catalepsy, hypothermia, and analgesia. Common assays for these behavioral paradigms are as follows:

Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system, which is involved in a variety of physiological processes including appetite, pain-sensation, mood, and memory.
- Spontaneous activity (or hypomotility) is determined by an open field test, in which a mouse is placed in a cage with perpendicular grid lines, usually spaced by approx. 1 inch. An experimenter counts the number of line crossings by the mouse in a given amount of time.
- Catalepsy is determined by the bar test. The mouse is placed on a bar oriented parallel to and approximately 1 inch off of the ground. If the mouse remains immobile on the bar for typically more than 20 seconds, it is considered cataleptic.
- Hypothermia (reduced body temperature) is determined by using a rectal probe to measure the rectal temperature.
- Analgesia is usually determined by the hot plate or tail immersion test. In the hot plate test, the mouse is placed on a heated plate, typically between 54 and 58°C. An experimenter measures the time it takes for the animal to raise its feet or jump off of the hot plate. In the tail immersion test, the mouse is immobilized and its tail is placed into a warm water bath, typically also between 54 and 58°C. An experimenter measures the time it takes for the mouse to remove its tail from the water bath.
Developed by Calvin S. Hall, the open field test is an experimental test used to assay general locomotor activity levels, anxiety, and willingness to explore in animals in scientific research. However, the extent to which behavior in the open field measures anxiety is controversial.
Catalepsy is a nervous condition characterized by muscular rigidity and fixity of posture regardless of external stimuli, as well as decreased sensitivity to pain.

Hypothermia is defined as a body core temperature below 35.0 °C (95.0 °F) in humans. Symptoms depend on the temperature. In mild hypothermia there is shivering and mental confusion. In moderate hypothermia shivering stops and confusion increases. In severe hypothermia, there may be paradoxical undressing, in which a person removes their clothing, as well as an increased risk of the heart stopping.
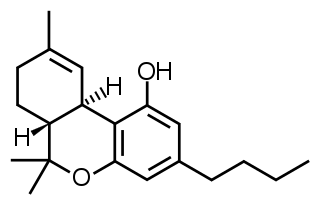
Direct CB1 agonists, such as THC (the psychoactive component of marijuana), or WIN 55,212-2, have effects in all components of the tetrad and induce hypomotility, catalepsy, hypothermia, and analgesia in rodents. Accordingly, all true "tetrad effects" are not observed following treatment with antagonists or inverse agonists of CB1 such as rimonabant. Data have shown, that also CB2 receptors are involved in the tetrad effects induced by cannabinoids, and other, associated with CB1 agonism. [3]
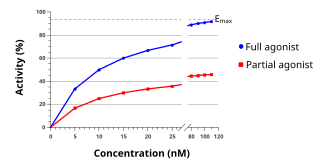
An agonist is a chemical that binds to a receptor and activates the receptor to produce a biological response. Whereas an agonist causes an action, an antagonist blocks the action of the agonist, and an inverse agonist causes an action opposite to that of the agonist.

WIN 55,212-2 is a chemical described as an aminoalkylindole derivative, which produces effects similar to those of cannabinoids such as tetrahydrocannabinol (THC) but has an entirely different chemical structure.

A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. They are sometimes called blockers; examples include alpha blockers, beta blockers, and calcium channel blockers. In pharmacology, antagonists have affinity but no efficacy for their cognate receptors, and binding will disrupt the interaction and inhibit the function of an agonist or inverse agonist at receptors. Antagonists mediate their effects by binding to the active site or to the allosteric site on a receptor, or they may interact at unique binding sites not normally involved in the biological regulation of the receptor's activity. Antagonist activity may be reversible or irreversible depending on the longevity of the antagonist–receptor complex, which, in turn, depends on the nature of antagonist–receptor binding. The majority of drug antagonists achieve their potency by competing with endogenous ligands or substrates at structurally defined binding sites on receptors.