
Interferons are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses.

A poliovirus, the causative agent of polio, is a serotype of the species Enterovirus C, in the family of Picornaviridae. There are three poliovirus serotypes: types 1, 2, and 3.

Japanese encephalitis (JE) is an infection of the brain caused by the Japanese encephalitis virus (JEV). While most infections result in little or no symptoms, occasional inflammation of the brain occurs. In these cases, symptoms may include headache, vomiting, fever, confusion and seizures. This occurs about 5 to 15 days after infection.

Max Theiler was a South African-American virologist and physician. He was awarded the Nobel Prize in Physiology or Medicine in 1951 for developing a vaccine against yellow fever in 1937, becoming the first African-born Nobel laureate.
Experimental autoimmune encephalomyelitis, sometimes experimental allergic encephalomyelitis (EAE), is an animal model of brain inflammation. It is an inflammatory demyelinating disease of the central nervous system (CNS). It is mostly used with rodents and is widely studied as an animal model of the human CNS demyelinating diseases, including multiple sclerosis (MS) and acute disseminated encephalomyelitis (ADEM). EAE is also the prototype for T-cell-mediated autoimmune disease in general.

Multiple sclerosis is an inflammatory demyelinating disease of the CNS in which activated immune cells invade the central nervous system and cause inflammation, neurodegeneration, and tissue damage. The underlying cause is currently unknown. Current research in neuropathology, neuroimmunology, neurobiology, and neuroimaging, together with clinical neurology, provide support for the notion that MS is not a single disease but rather a spectrum.

Interferon regulatory factors (IRF) are proteins which regulate transcription of interferons. Interferon regulatory factors contain a conserved N-terminal region of about 120 amino acids, which folds into a structure that binds specifically to the IRF-element (IRF-E) motifs, which is located upstream of the interferon genes. Some viruses have evolved defense mechanisms that regulate and interfere with IRF functions to escape the host immune system. For instance, the remaining parts of the interferon regulatory factor sequence vary depending on the precise function of the protein. The Kaposi sarcoma herpesvirus, KSHV, is a cancer virus that encodes four different IRF-like genes; including vIRF1, which is a transforming oncoprotein that inhibits type 1 interferon activity. In addition, the expression of IRF genes is under epigenetic regulation by promoter DNA methylation.
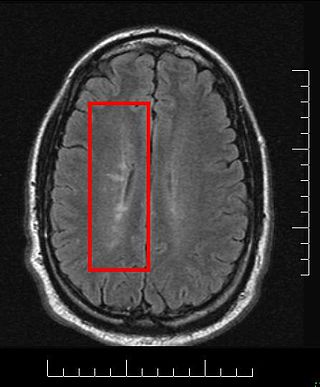
Multiple sclerosis and other demyelinating diseases of the central nervous system (CNS) produce lesions and glial scars or scleroses. They present different shapes and histological findings according to the underlying condition that produces them.

Murine coronavirus (M-CoV) is a virus in the genus Betacoronavirus that infects mice. Belonging to the subgenus Embecovirus, murine coronavirus strains are enterotropic or polytropic. Enterotropic strains include mouse hepatitis virus (MHV) strains D, Y, RI, and DVIM, whereas polytropic strains, such as JHM and A59, primarily cause hepatitis, enteritis, and encephalitis. Murine coronavirus is an important pathogen in the laboratory mouse and the laboratory rat. It is the most studied coronavirus in animals other than humans, and has been used as an animal disease model for many virological and clinical studies.
Sulfatide, also known as 3-O-sulfogalactosylceramide, SM4, or sulfated galactocerebroside, is a class of sulfolipids, specifically a class of sulfoglycolipids, which are glycolipids that contain a sulfate group. Sulfatide is synthesized primarily starting in the endoplasmic reticulum and ending in the Golgi apparatus where ceramide is converted to galactocerebroside and later sulfated to make sulfatide. Of all of the galactolipids that are found in the myelin sheath, one fifth of them are sulfatide. Sulfatide is primarily found on the extracellular leaflet of the myelin plasma membrane produced by the oligodendrocytes in the central nervous system and in the Schwann cells in the peripheral nervous system. However, sulfatide is also present on the extracellular leaflet of the plasma membrane of many cells in eukaryotic organisms.

Murine respirovirus, formerly Sendai virus (SeV) and previously also known as murine parainfluenza virus type 1 or hemagglutinating virus of Japan (HVJ), is an enveloped, 150-200 nm–diameter, negative sense, single-stranded RNA virus of the family Paramyxoviridae. It typically infects rodents and it is not pathogenic for humans or domestic animals. Sendai virus (SeV) is a member of the genus Respirovirus. The virus was isolated in the city of Sendai in Japan in the early 1950s. Since then, it has been actively used in research as a model pathogen. The virus is infectious for many cancer cell lines, and has oncolytic properties demonstrated in animal models and in naturally-occurring cancers in animals. SeV's ability to fuse eukaryotic cells and to form syncytium was used to produce hybridoma cells capable of manufacturing monoclonal antibodies in large quantities. Recent applications of SeV-based vectors include the reprogramming of somatic cells into induced pluripotent stem cells and vaccine creation. For vaccination purpose the Sendai virus-based constructs could be delivered in a form of nasal drops, which may be beneficial in inducing a mucosal immune response. SeV has several features that are important in a vector for a successful vaccine: the virus does not integrate into the host genome, it does not undergo genetic recombination, it replicates only in the cytoplasm without DNA intermediates or a nuclear phase and it is does not cause any disease in humans or domestic animals. Sendai virus is used as a backbone for vaccine development against Mycobacterium tuberculosis that causes tuberculosis, against HIV-1 that causes AIDS and against other viruses, including those that cause severe respiratory infections in children. The latter include Human Respiratory Syncytial Virus (HRSV), Human Metapneumovirus (HMPV) and Human Parainfluenza Viruses (HPIV). The vaccine studies against M. tuberculosis, HMPV, HPIV1 and, HPIV2 are in the pre-clinical stage, against HRSV a phase I clinical trail has been completed. The phase I clinical studies of SeV-based vaccination were also completed for HPIV1. They were done in adults and in 3- to 6-year-old children. As a result of vaccination against HPIV1 a significant boost in virus-specific neutralizing antibodies was observed. A SeV-based vaccine development against HIV-1 has reached a phase II clinical trial. In Japan intranasal Sendai virus-based SARS-CoV-2 vaccine was created and tested in a mouse model.

Interferon regulatory factor 3, also known as IRF3, is an interferon regulatory factor.

Signal transducer and activator of transcription 4 (STAT4) is a transcription factor belonging to the STAT protein family, composed of STAT1, STAT2, STAT3, STAT5A, STAT5B, STAT6. STAT proteins are key activators of gene transcription which bind to DNA in response to cytokine gradient. STAT proteins are a common part of Janus kinase (JAK)- signalling pathways, activated by cytokines.STAT4 is required for the development of Th1 cells from naive CD4+ T cells and IFN-γ production in response to IL-12. There are two known STAT4 transcripts, STAT4α and STAT4β, differing in the levels of interferon-gamma production downstream.

Signal transducer and activator of transcription 2 is a protein that in humans is encoded by the STAT2 gene. It is a member of the STAT protein family. This protein is critical to the biological response of type I interferons (IFNs). STAT2 sequence identity between mouse and human is only 68%.

Interferon regulatory factor 7, also known as IRF7, is a member of the interferon regulatory factor family of transcription factors.

Probable G-protein coupled receptor 84 is a protein that in humans is encoded by the GPR84 gene.

Interferon regulatory factor 5 is a protein that in humans is encoded by the IRF5 gene. The IRF family is a group of transcription factors that are involved in signaling for virus responses in mammals along with regulation of certain cellular functions.

Stimulator of interferon genes (STING), also known as transmembrane protein 173 (TMEM173) and MPYS/MITA/ERIS is a protein that in humans is encoded by the STING1 gene.
Neuroinflammation is inflammation of the nervous tissue. It may be initiated in response to a variety of cues, including infection, traumatic brain injury, toxic metabolites, or autoimmunity. In the central nervous system (CNS), including the brain and spinal cord, microglia are the resident innate immune cells that are activated in response to these cues. The CNS is typically an immunologically privileged site because peripheral immune cells are generally blocked by the blood–brain barrier (BBB), a specialized structure composed of astrocytes and endothelial cells. However, circulating peripheral immune cells may surpass a compromised BBB and encounter neurons and glial cells expressing major histocompatibility complex molecules, perpetuating the immune response. Although the response is initiated to protect the central nervous system from the infectious agent, the effect may be toxic and widespread inflammation as well as further migration of leukocytes through the blood–brain barrier.
The cGAS–STING pathway is a component of the innate immune system that functions to detect the presence of cytosolic DNA and, in response, trigger expression of inflammatory genes that can lead to senescence or to the activation of defense mechanisms. DNA is normally found in the nucleus of the cell. Localization of DNA to the cytosol is associated with tumorigenesis, viral infection, and invasion by some intracellular bacteria. The cGAS – STING pathway acts to detect cytosolic DNA and induce an immune response.














