External links
| This chemistry-related article is a stub. You can help Wikipedia by expanding it. |
A liquid crystal (LC) is thermotropic if the order of its components[ clarify ] is determined or changed by temperature.
If temperature is too high, the rise in energy and therefore in motion of the components will induce a phase transition: the LC will become an isotropic liquid. If, on the contrary, temperature is too low to support a thermotropic phase, the LC will change to glass phase.
There is therefore a range of temperatures at which we observe thermotropic LCs; and most of these have several "subphases" (nematic, smectic...), which we may observe by modifying the temperature.
| This chemistry-related article is a stub. You can help Wikipedia by expanding it. |
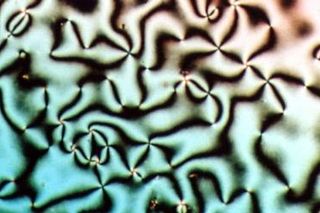
Liquid crystals (LCs) are a state of matter which has properties between those of conventional liquids and those of solid crystals. For instance, a liquid crystal may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of liquid-crystal phases, which can be distinguished by their different optical properties. The contrasting areas in the textures correspond to domains where the liquid-crystal molecules are oriented in different directions. Within a domain, however, the molecules are well ordered. LC materials may not always be in a liquid-crystal state of matter.

In the physical sciences, a phase is a region of space, throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is another separate phase.

In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many intermediate states are known to exist, such as liquid crystal, and some states only exist under extreme conditions, such as Bose–Einstein condensates, neutron-degenerate matter, and quark–gluon plasma, which only occur, respectively, in situations of extreme cold, extreme density, and extremely high energy. For a complete list of all exotic states of matter, see the list of states of matter.

The melting point of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa.

In chemistry, thermodynamics, and many other related fields, phase transitions are the physical processes of transition between the basic states of matter: solid, liquid, and gas, as well as plasma in rare cases.
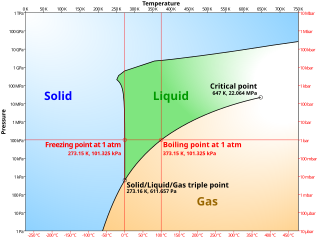
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases occur and coexist at equilibrium.

Solubility is the property of a solid, liquid or gaseous chemical substance called solute to dissolve in a solid, liquid or gaseous solvent. The solubility of a substance fundamentally depends on the physical and chemical properties of the solute and solvent as well as on temperature, pressure and presence of other chemicals of the solution. The extent of the solubility of a substance in a specific solvent is measured as the saturation concentration, where adding more solute does not increase the concentration of the solution and begins to precipitate the excess amount of solute.
In physics and chemistry, flash freezing is the process whereby objects are frozen in just a few hours by subjecting them to cryogenic temperatures, or through direct contact with liquid nitrogen at −196 °C (−320.8 °F). It is commonly used in the food industry.
A mesogen is a compound that displays liquid crystal properties. Mesogens can be described as disordered solids or ordered liquids because they arise from a unique state of matter that exhibits both solid- and liquid-like properties called the liquid crystalline state. This liquid crystalline state (LC) is called the mesophase and occurs between the crystalline solid (Cr) state and the isotropic liquid (Iso) state at distinct temperature ranges.
A biaxial nematic is a spatially homogeneous liquid crystal with three distinct optical axes. This is to be contrasted to a simple nematic, which has a single preferred axis, around which the system is rotationally symmetric. The symmetry group of a biaxial nematic is i.e. that of a rectangular right parallelepiped, having 3 orthogonal axes and three orthogonal mirror planes. In a frame co-aligned with optical axes the second rank order parameter tensor of a biaxial nematic has the form

Crystallization or crystallisation is the process by which a solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some of the ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas. Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, and in the case of liquid crystals, time of fluid evaporation.

Thermochromism is the property of substances to change color due to a change in temperature. A mood ring is an excellent example of this phenomenon, but thermochromism also has more practical uses, such as baby bottles which change to a different color when cool enough to drink, or kettles which change when water is at or near boiling point. Thermochromism is one of several types of chromism.

Nucleation is the first step in the formation of either a new thermodynamic phase or a new structure via self-assembly or self-organization. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. For example, if a volume of water is cooled below 0 °C, it will tend to freeze into ice, but volumes of water cooled only a few degrees below 0 °C often stay completely free of ice for long periods. At these conditions, nucleation of ice is either slow or does not occur at all. However, at lower temperatures ice crystals appear after little or no delay. At these conditions ice nucleation is fast. Nucleation is commonly how first-order phase transitions start, and then it is the start of the process of forming a new thermodynamic phase. In contrast, new phases at continuous phase transitions start to form immediately.
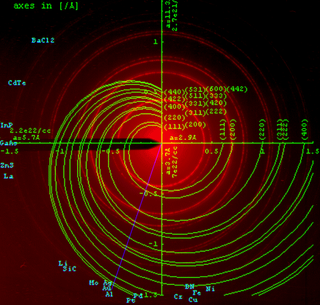
Powder diffraction is a scientific technique using X-ray, neutron, or electron diffraction on powder or microcrystalline samples for structural characterization of materials. An instrument dedicated to performing such powder measurements is called a powder diffractometer.
A liquid crystal thermometer, temperature strip or plastic strip thermometer is a type of thermometer that contains heat-sensitive (thermochromic) liquid crystals in a plastic strip that change colour to indicate different temperatures. Liquid crystals possess the mechanical properties of a liquid, but have the optical properties of a single crystal. Temperature changes can affect the colour of a liquid crystal, which makes them useful for temperature measurement. The resolution of liquid crystal sensors is in the 0.1°C range. Disposable liquid crystal thermometers have been developed for home and medical use. For example if the thermometer is black and it is put onto someone's forehead it will change colour depending on the temperature of the person.
Liquid-crystal polymers (LCPs) are a class of aromatic polymers. They are extremely unreactive and inert, and highly resistant to fire.

A liquid crystalline mesophase is called lyotropic if formed by dissolving an amphiphilic mesogen in a suitable solvent, under appropriate conditions of concentration, temperature and pressure. A mixture of soap and water is an everyday example of a lyotropic liquid crystal.
A blue phase mode LCD is a liquid crystal display (LCD) technology that uses highly twisted cholesteric phases in a blue phase. It was first proposed in 2007 to obtain a better display of moving images with, for example, frame rates of 100–120 Hz to improve the temporal response of LCDs. This operational mode for LCDs also does not require anisotropic alignment layers and thus theoretically simplifies the LCD manufacturing process.
There are various classifications of the electro-optical modes of liquid crystal displays (LCDs).

Temperature sensitive glass is a glass material that reacts to ambient temperatures radiated off of other surfaces, e.g. hands or water. The liquid crystals beneath the glass surface impact color upon temperature. There are three main phases of these crystals: nematic, smectic, and chiral.