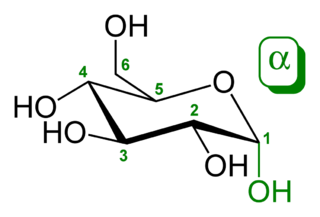
Glucose (also called dextrose) is a simple sugar with the molecular formula C6H12O6. Glucose is the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight. There it is used to make cellulose in cell walls, which is the most abundant carbohydrate. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is partially stored as a polymer, in plants mainly as starch and amylopectin and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form of glucose is D-glucose, while L-glucose is produced synthetically in comparably small amounts and is of lesser importance.

Quercus petraea, commonly known as the sessile oak, Cornish oak, or durmast oak, is a species of oak tree native to most of Europe and into Anatolia and Iran.
A glucoside is a glycoside that is derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes.

Quercus macrocarpa, the bur oak, sometimes spelled burr oak, is a species of oak in the white oak section Quercus sect. Quercus, native to North America in the eastern and central United States and eastern and central Canada. This plant is also called mossycup oak and mossycup white oak.
Glycated hemoglobin (hemoglobin A1c, HbA1c, A1C, or less commonly HgbA1c, haemoglobin A1c, HbA1c, Hb1c, etc.) is a form of hemoglobin that is covalently bound to glucose. It is formed in a non-enzymatic glycation pathway by hemoglobin's exposure to plasma glucose. It is measured primarily to identify the three-month average plasma glucose concentration and thus can be used as a diagnostic test for diabetes and as assessment test for glycemic control in people with diabetes. The test is limited to a three-month average because the lifespan of a red blood cell is four months (120 days). However, since red blood cells do not all undergo lysis at the same time, HbA1C is taken as a limited measure of three months. HbA1c is a measure of the beta-N-1-deoxy fructosyl component of hemoglobin. The origin of the naming derives from Hemoglobin type A being separated on cation exchange chromatography. The first fraction to separate, probably considered to be pure Hemoglobin A, was designated HbA0, the following fractions were designated HbA1a, HbA1b, and HbA1c, respective of their order of elution. There have subsequently been many more sub fractions as separation techniques have improved. Normal levels of glucose produce a normal amount of glycated hemoglobin. As the average amount of plasma glucose increases, the fraction of glycated hemoglobin increases in a predictable way. This serves as an indicator that blood sugar is increasing and that action should be taken.

Ellagic acid is a natural phenol antioxidant found in numerous fruits and vegetables. The antiproliferative and antioxidant properties of ellagic acid have prompted research into its potential health benefits. Ellagic acid is the dilactone of hexahydroxydiphenic acid.

Quercitrin is a glycoside formed from the flavonoid quercetin and the deoxy sugar rhamnose.

Orientin is a flavone, a chemical flavonoid-like compound. It is the 8-C glucoside of luteolin.
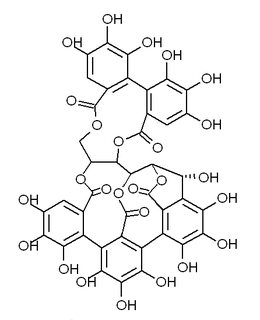
Castalagin is an ellagitannin, a type of hydrolyzable tannin, found in oak and chestnut wood and in the stem barks of Anogeissus leiocarpus and Terminalia avicennoides.
A hydrolyzable tannin or pyrogallol-type tannin is a type of tannin that, on heating with hydrochloric or sulfuric acids, yields gallic or ellagic acids.

Grandinin is an ellagitannin. It can be found in Melaleuca quinquenervia leaves and in oaks species like the North American white oak and European red oak. It shows antioxydant activity. It is an astringent compound. It is also found in wine, red or white, aged in oak barrels.

Glucogallin is chemical compound formed from gallic acid and β-D-glucose. It can be found in oaks species like the North American white oak, European red oak and Amla fruit.
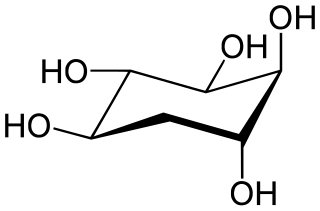
5-Deoxyinositol (quercitol) is a cyclitol. It can be found in wines aged in oak wood barrels. It can also be found in Quercus sp. (oaks) and in Gymnema sylvestre. It is different from quercetol, a synonym of quercetin.
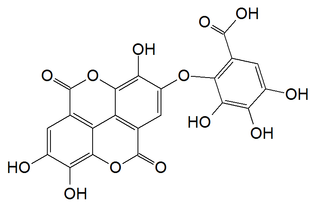
Valoneic acid dilactone is a hydrolysable tannin that can be isolated from the heartwood of Shorea laeviforia and in oaks species like the North American white oak and European red oak.

1,2,6-Trigalloyl glucose is a gallotannin found in cell cultures of Cornus officinalis.

1-O,6-O-Digalloyl-β-D-glucose is a gallotannin. It can be found in some oak species.
Digalloyl glucose may refer to:















