Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.

The aldol reaction is a means of forming carbon–carbon bonds in organic chemistry. Discovered independently by the Russian chemist Alexander Borodin in 1869 and by the French chemist Charles-Adolphe Wurtz in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound. These products are known as aldols, from the aldehyde + alcohol, a structural motif seen in many of the products. Aldol structural units are found in many important molecules, whether naturally occurring or synthetic. For example, the aldol reaction has been used in the large-scale production of the commodity chemical pentaerythritol and the synthesis of the heart disease drug Lipitor.

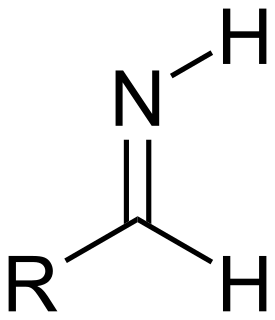
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen (H) or an organic group (R). If this group is not a hydrogen atom, then the compound can sometimes be referred to as a Schiff base. The carbon atom has two additional single bonds. The term "imine" was coined in 1883 by the German chemist Albert Ladenburg.
The Fischer indole synthesis is a chemical reaction that produces the aromatic heterocycle indole from a (substituted) phenylhydrazine and an aldehyde or ketone under acidic conditions. The reaction was discovered in 1883 by Emil Fischer. Today antimigraine drugs of the triptan class are often synthesized by this method.

Imidazole is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms.

Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a pKa of 0.8, compared to 7 for imidazole.
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen; the term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1).
The Robinson annulation is a chemical reaction used in organic chemistry for ring formation. It was discovered by Robert Robinson in 1935 as a method to create a six membered ring by forming three new carbon–carbon bonds. The method uses a ketone and a methyl vinyl ketone to form an α,β-unsaturated ketone in a cyclohexane ring by a Michael addition followed by an aldol condensation. This procedure is one of the key methods to form fused ring systems.
The Ugi reaction is a multi-component reaction in organic chemistry involving a ketone or aldehyde, an amine, an isocyanide and a carboxylic acid to form a bis-amide. The reaction is named after Ivar Karl Ugi, who first reported this reaction in 1959.

The Fischer oxazole synthesis is a chemical synthesis of an oxazole from a cyanohydrin and an aldehyde in the presence of anhydrous hydrochloric acid. This method was discovered by Emil Fischer in 1896. The cyanohydrin itself is derived from a separate aldehyde. The reactants of the oxazole synthesis itself, the cyanohydrin of an aldehyde and the other aldehyde itself, are usually present in equimolar amounts. Both reactants usually have an aromatic group, which appear at specific positions on the resulting heterocycle.
The Reformatsky reaction is an organic reaction which condenses aldehydes or ketones, with α-halo esters, using a metallic zinc to form β-hydroxy-esters:

The Nicolaou Taxol total synthesis, published by K. C. Nicolaou and his group in 1994 concerns the total synthesis of Taxol. Taxol is an important drug in the treatment of cancer but also expensive because the compound is harvested from a scarce resource, namely the pacific yew.
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, was discovered by German chemist Adolph Strecker, and is a term used for a series of chemical reactions that synthesize an amino acid from an aldehyde or ketone. The aldehyde is condensed with ammonium chloride in the presence of potassium cyanide to form an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino acid. In the original Strecker reaction acetaldehyde, ammonia, and hydrogen cyanide combined to form after hydrolysis alanine.

The Seyferth–Gilbert homologation is a chemical reaction of an aryl ketone 1 with dimethyl (diazomethyl)phosphonate 2 and potassium tert-butoxide to give substituted alkynes 3. Dimethyl (diazomethyl)phosphonate 2 is often called the Seyferth–Gilbert reagent.

The Dakin oxidation is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde or ketone reacts with hydrogen peroxide in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidized, and the hydrogen peroxide is reduced.

The article concerns the total synthesis of galanthamine, a drug used for the treatment of mild to moderate Alzheimer's disease.
A homologation reaction, also known as homologization, is any chemical reaction that converts the reactant into the next member of the homologous series. A homologous series is a group of compounds that differ by a constant unit, generally a (-CH2-) group. The reactants undergo a homologation when the number of a repeated structural unit in the molecules is increased. The most common homologation reactions increase the number of methylene (-CH2-) units in saturated chain within the molecule. For example, the reaction of aldehydes or ketones with diazomethane or methoxymethylenetriphenylphosphine to give the next homologue in the series.
The α-ketol rearrangement is the acid-, base-, or heat-induced 1,2-migration of an alkyl or aryl group in an α-hydroxy ketone or aldehyde to give an isomeric product.