Related Research Articles

A Carnot heat engine is a heat engine that operates on the Carnot cycle. The basic model for this engine was developed by Nicolas Léonard Sadi Carnot in 1824. The Carnot engine model was graphically expanded by Benoît Paul Émile Clapeyron in 1834 and mathematically explored by Rudolf Clausius in 1857, work that led to the fundamental thermodynamic concept of entropy. The Carnot engine is the most efficient heat engine which is theoretically possible. The efficiency depends only upon the absolute temperatures of the hot and cold heat reservoirs between which it operates.

In thermodynamics and engineering, a heat engine is a system that converts heat to mechanical energy, which can then be used to do mechanical work. It does this by bringing a working substance from a higher state temperature to a lower state temperature. A heat source generates thermal energy that brings the working substance to the higher temperature state. The working substance generates work in the working body of the engine while transferring heat to the colder sink until it reaches a lower temperature state. During this process some of the thermal energy is converted into work by exploiting the properties of the working substance. The working substance can be any system with a non-zero heat capacity, but it usually is a gas or liquid. During this process, some heat is normally lost to the surroundings and is not converted to work. Also, some energy is unusable because of friction and drag.

A simple machine is a mechanical device that changes the direction or magnitude of a force. In general, they can be defined as the simplest mechanisms that use mechanical advantage to multiply force. Usually the term refers to the six classical simple machines that were defined by Renaissance scientists:

An inclined plane, also known as a ramp, is a flat supporting surface tilted at an angle, with one end higher than the other, used as an aid for raising or lowering a load. The inclined plane is one of the six classical simple machines defined by Renaissance scientists. Inclined planes are used to move heavy loads over vertical obstacles. Examples vary from a ramp used to load goods into a truck, to a person walking up a pedestrian ramp, to an automobile or railroad train climbing a grade.

A Stirling engine is a heat engine that is operated by the cyclic compression and expansion of air or other gas between different temperatures, resulting in a net conversion of heat energy to mechanical work.

The Stirling cycle is a thermodynamic cycle that describes the general class of Stirling devices. This includes the original Stirling engine that was invented, developed and patented in 1816 by Robert Stirling with help from his brother, an engineer.
Efficiency is the ability to avoid wasting materials, energy, efforts, money, and time in doing something or in producing a desired result. In a more general sense, it is the ability to do things well, successfully, and without waste. "Efficiency is thus not a goal in itself. It is not something we want for its own sake, but rather because it helps us attain more of the things we value". In more mathematical or scientific terms, it signifies the level of performance that uses the least amount of inputs to achieve the highest amount of output. It often specifically comprises the capability of a specific application of effort to produce a specific outcome with a minimum amount or quantity of waste, expense, or unnecessary effort. Efficiency refers to very different inputs and outputs in different fields and industries.

Carnot's theorem, developed in 1824 by Nicolas Léonard Sadi Carnot, also called Carnot's rule, is a principle that specifies limits on the maximum efficiency that any heat engine can obtain.

Fuel efficiency is a form of thermal efficiency, meaning the ratio of effort to result of a process that converts chemical potential energy contained in a carrier (fuel) into kinetic energy or work. Overall fuel efficiency may vary per device, which in turn may vary per application, and this spectrum of variance is often illustrated as a continuous energy profile. Non-transportation applications, such as industry, benefit from increased fuel efficiency, especially fossil fuel power plants or industries dealing with combustion, such as ammonia production during the Haber process.
In mechanical engineering, mechanical efficiency is a dimensionless number that measures the effectiveness of a mechanism or machine in transforming the power input to the device to power output. A machine is a mechanical linkage in which force is applied at one point, and the force does work moving a load at another point. At any instant the power input to a machine is equal to the input force multiplied by the velocity of the input point, similarly the power output is equal to the force exerted on the load multiplied by the velocity of the load. The mechanical efficiency of a machine is a dimensionless number between 0 and 1 that is the ratio between the power output of the machine and the power input
The coefficient of performance or COP of a heat pump, refrigerator or air conditioning system is a ratio of useful heating or cooling provided to work (energy) required. Higher COPs equate to higher efficiency, lower energy (power) consumption and thus lower operating costs. The COP usually exceeds 1, especially in heat pumps, because, instead of just converting work to heat, it pumps additional heat from a heat source to where the heat is required. Most air conditioners have a COP of 2.3 to 3.5. Less work is required to move heat than for conversion into heat, and because of this, heat pumps, air conditioners and refrigeration systems can have a coefficient of performance greater than one. However, this does not mean that they are more than 100% efficient, in other words, no heat engine can have a thermal efficiency of 100% or greater. For complete systems, COP calculations should include energy consumption of all power consuming auxiliaries. The COP is highly dependent on operating conditions, especially absolute temperature and relative temperature between sink and system, and is often graphed or averaged against expected conditions. Performance of absorption refrigerator chillers is typically much lower, as they are not heat pumps relying on compression, but instead rely on chemical reactions driven by heat.

The Rankine cycle is an idealized thermodynamic cycle describing the process by which certain heat engines, such as steam turbines or reciprocating steam engines, allow mechanical work to be extracted from a fluid as it moves between a heat source and heat sink. The Rankine cycle is named after William John Macquorn Rankine, a Scottish polymath professor at Glasgow University.
For fluid power, a working fluid is a gas or liquid that primarily transfers force, motion, or mechanical energy. In hydraulics, water or hydraulic fluid transfers force between hydraulic components such as hydraulic pumps, hydraulic cylinders, and hydraulic motors that are assembled into hydraulic machinery, hydraulic drive systems, etc. In pneumatics, the working fluid is air or another gas which transfers force between pneumatic components such as compressors, vacuum pumps, pneumatic cylinders, and pneumatic motors. In pneumatic systems, the working gas also stores energy because it is compressible.
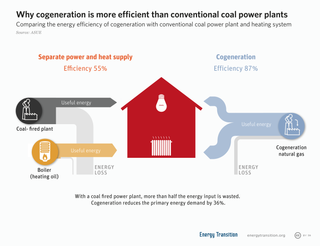
Cogeneration or combined heat and power (CHP) is the use of a heat engine or power station to generate electricity and useful heat at the same time.
In thermodynamics, the exergy of a system is the maximum useful work possible during a process that brings the system into equilibrium with a heat reservoir, reaching maximum entropy. When the surroundings are the reservoir, exergy is the potential of a system to cause a change as it achieves equilibrium with its environment. Exergy is the energy that is available to be used. After the system and surroundings reach equilibrium, the exergy is zero. Determining exergy was also the first goal of thermodynamics. The term "exergy" was coined in 1956 by Zoran Rant (1904–1972) by using the Greek ex and ergon meaning "from work", but the concept had been earlier developed by J Willard Gibbs in 1873.

Motor drive means a system that includes a motor. An adjustable speed motor drive means a system that includes a motor that has multiple operating speeds. A variable speed motor drive is a system that includes a motor and is continuously variable in speed. If the motor is generating electrical energy rather than using it – this could be called a generator drive but is often still referred to as a motor drive.

A thermodynamic cycle consists of a linked sequence of thermodynamic processes that involve transfer of heat and work into and out of the system, while varying pressure, temperature, and other state variables within the system, and that eventually returns the system to its initial state. In the process of passing through a cycle, the working fluid (system) may convert heat from a warm source into useful work, and dispose of the remaining heat to a cold sink, thereby acting as a heat engine. Conversely, the cycle may be reversed and use work to move heat from a cold source and transfer it to a warm sink thereby acting as a heat pump. At every point in the cycle, the system is in thermodynamic equilibrium, so the cycle is reversible.

In thermodynamics, the thermal efficiency is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, steam turbine, steam engine, boiler, furnace, refrigerator, ACs etc.

A Carnot cycle is an ideal thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. It provides an upper limit on the efficiency of any classical thermodynamic engine during the conversion of heat into work, or conversely, the efficiency of a refrigeration system in creating a temperature difference through the application of work to the system.

Thermodynamic heat pump cycles or refrigeration cycles are the conceptual and mathematical models for heat pump, air conditioning and refrigeration systems. A heat pump is a mechanical system that allows for the transmission of heat from one location at a lower temperature to another location at a higher temperature. Thus a heat pump may be thought of as a "heater" if the objective is to warm the heat sink, or a "refrigerator" or “cooler” if the objective is to cool the heat source. In either case, the operating principles are similar. Heat is moved from a cold place to a warm place.
References
- NewPath Learning (1 March 2014). Work, Power & Simple Machines Science Learning Guide. NewPath Learning. pp. 8–. ISBN 978-1-63212-074-8.