Related Research Articles

Pfizer Inc. is an American multinational pharmaceutical and biotechnology corporation headquartered on 42nd Street in Manhattan, New York City. The company was established in 1849 in New York by two German entrepreneurs, Charles Pfizer (1824–1906) and his cousin Charles F. Erhart (1821–1891).

AstraZeneca plc is a British-Swedish multinational pharmaceutical and biotechnology company with its headquarters at the Cambridge Biomedical Campus in Cambridge, England. It has a portfolio of products for major diseases in areas including oncology, cardiovascular, gastrointestinal, infection, neuroscience, respiratory, and inflammation. It has been involved in developing the Oxford–AstraZeneca COVID-19 vaccine.

F. Hoffmann-La Roche AG, commonly known as Roche, is a Swiss multinational healthcare company that operates worldwide under two divisions: Pharmaceuticals and Diagnostics. Its holding company, Roche Holding AG, has shares listed on the SIX Swiss Exchange. The company headquarters are located in Basel. Roche is the fifth largest pharmaceutical company in the world by revenue, and the leading provider of cancer treatments globally.

Cefpodoxime is an oral, third-generation cephalosporin antibiotic available in the US in various generic preparations. It is active against both Gram-positive and Gram-negative organisms with notable exceptions including Pseudomonas aeruginosa, Enterococcus, and Bacteroides fragilis. It is typically used to treat acute otitis media, pharyngitis, sinusitis, and gonorrhea. It also finds use as oral continuation therapy when intravenous cephalosporins are no longer necessary for continued treatment.
Forest Laboratories was a company in the pharmaceutical industry incorporated in Delaware, with its principal office in New York City. It was known for licensing European pharmaceuticals for sale in the United States. On July 1, 2014, the company was acquired by Actavis.
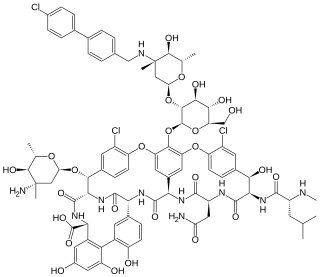
Oritavancin, sold under the brand name Orbactiv among others, is a semisynthetic glycopeptide antibiotic medication for the treatment of serious Gram-positive bacterial infections. Its chemical structure as a lipoglycopeptide is similar to vancomycin.
Targanta Therapeutics Corporation was a biopharmaceutical company headquartered in Cambridge, Massachusetts. The company also had operations in Indianapolis, Montreal and Toronto. Targanta completed its initial public offering on October 9, 2007 and traded on the Nasdaq market under the symbol: TARG. Targanta was acquired by The Medicines Company in 2009.

Dalbavancin, sold under the brand names Dalvance in the US and Xydalba in the EU among others, is a second-generation lipoglycopeptide antibiotic medication. It belongs to the same class as vancomycin, the most widely used and one of the treatments available to people infected with methicillin-resistant Staphylococcus aureus (MRSA).

Cefovecin (INN) is an antibiotic of the cephalosporin class, licensed for the treatment of skin infections in cats and dogs. It is marketed by Zoetis under the trade name Convenia. It is used to treat skin infections caused by Pasteurella multocida in cats, and Staphylococcus intermedius and Streptococcus canis in dogs. The advantage of using a long-acting injectable antibiotic is that, unlike with daily administration, doses cannot be missed, which may allow partially resistant microbes to recover. The disadvantage is the presence of subtherapeutic concentrations in the weeks after the resolution of infections. This is associated with the development of resistance in microbes. It should not be used in pregnant or lactating animals or in animals with a history of allergies to penicillin or cephalosporin drugs.

Iclaprim is an antibiotic drug candidate that is active against Gram positive organisms. It is administered intravenously.
Melinta Therapeutics, founded in 2000 as Rib-X Pharmaceuticals, is an American publicly traded biopharmaceutical firm that focuses on the design and development of novel broad-spectrum antibiotics for the treatment of antibiotic-resistant infections in hospital and community settings. The company is located in New Haven, Connecticut.
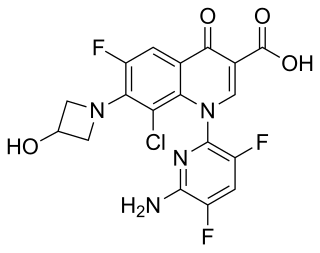
Delafloxacin sold under the brand name Baxdela among others, is a fluoroquinolone antibiotic used to treat acute bacterial skin and skin structure infections.

Tedizolid, is an oxazolidinone-class antibiotic. Tedizolid phosphate is a phosphate ester prodrug of the active compound tedizolid. It was developed by Cubist Pharmaceuticals, following acquisition of Trius Therapeutics, and is marketed for the treatment of acute bacterial skin and skin structure infections.

Ceftaroline fosamil (INN), brand name Teflaro in the US and Zinforo in Europe, is a cephalosporin antibiotic with anti-MRSA activity. Ceftaroline fosamil is a prodrug of ceftaroline. It is active against methicillin-resistant Staphylococcus aureus (MRSA) and other Gram-positive bacteria. It retains some activity of later-generation cephalosporins having broad-spectrum activity against Gram-negative bacteria, but its effectiveness is relatively much weaker. It is currently being investigated for community-acquired pneumonia and complicated skin and skin structure infection.

Lipoglycopeptides are a class of antibiotic that have lipophilic side-chains linked to glycopeptides. The class includes oritavancin, telavancin and dalbavancin.
Taksta is a front-loaded oral dosing regimen of sodium fusidate under development in the U.S. as an antibiotic for gram-positive infections including drug-resistant strains such as methicillin-resistant Staphylococcus aureus (MRSA).
Trius Therapeutics was a biopharma company based in San Diego, CA that focused on the development of antibiotics.

Omadacycline, sold under the brand name Nuzyra, is a broad spectrum antibiotic medication belonging to the aminomethylcycline subclass of tetracycline antibiotics. In the United States, it was approved in October 2018, for the treatment of community-acquired bacterial pneumonia and acute skin and skin structure infections.
Brilacidin, an investigational new drug (IND), is a polymer-based antibiotic currently in human clinical trials, and represents a new class of antibiotics called host defense protein mimetics, or HDP-mimetics, which are non-peptide synthetic small molecules modeled after host defense peptides (HDPs). HDPs, also called antimicrobial peptides, some of which are defensins, are part of the innate immune response and are common to most higher forms of life. As brilacidin is modeled after a defensin, it is also called a defensin mimetic.
Seagen Inc. is an American biotechnology company focused on developing and commercializing innovative, empowered monoclonal antibody-based therapies for the treatment of cancer. The company, headquartered in Bothell, Washington, is the industry leader in antibody-drug conjugates or ADCs, a technology designed to harness the targeting ability of monoclonal antibodies to deliver cell-killing agents directly to cancer cells. Antibody-drug conjugates are intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy, while potentially enhancing antitumor activity.
References
- ↑ "Newly Formed Durata Therapeutics Acquires Pfizer's Vicuron Subsidiary" (Press release). PR Newswire. December 21, 2009.
- ↑ "Durata Therapeutics Initiates Phase 3 Study of Dalbavancin for the Treatment of Acute Bacterial Skin and Skin Structure Infections" (Press release). Business Wire. April 19, 2011.
- ↑ Frost, Peter (November 14, 2012). "Durata biotech moving headquarters to Chicago". Chicago Tribune .