
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body.

Sodium fluoride (NaF) is an inorganic compound with the formula NaF. It is a colorless or white solid that is readily soluble in water. It is used in trace amounts in the fluoridation of drinking water to prevent tooth decay, and in toothpastes and topical pharmaceuticals for the same purpose. In 2020, it was the 265th most commonly prescribed medication in the United States, with more than 1 million prescriptions. It is also used in metallurgy and in medical imaging.

A bone scan or bone scintigraphy is a nuclear medicine imaging technique of the bone. It can help diagnose a number of bone conditions, including cancer of the bone or metastasis, location of bone inflammation and fractures, and bone infection (osteomyelitis).

[18F]Fluorodeoxyglucose (INN), or fluorodeoxyglucose F 18, also commonly called fluorodeoxyglucose and abbreviated [18F]FDG, 2-[18F]FDG or FDG, is a radiopharmaceutical, specifically a radiotracer, used in the medical imaging modality positron emission tomography (PET). Chemically, it is 2-deoxy-2-[18F]fluoro-D-glucose, a glucose analog, with the positron-emitting radionuclide fluorine-18 substituted for the normal hydroxyl group at the C-2 position in the glucose molecule.
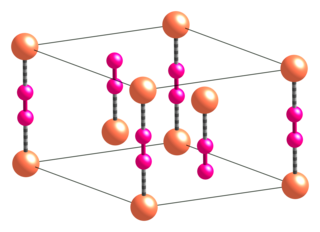
Copper(I) cyanide is an inorganic compound with the formula CuCN. This off-white solid occurs in two polymorphs; impure samples can be green due to the presence of Cu(II) impurities. The compound is useful as a catalyst, in electroplating copper, and as a reagent in the preparation of nitriles.
Oxygen-18 is a natural, stable isotope of oxygen and one of the environmental isotopes.
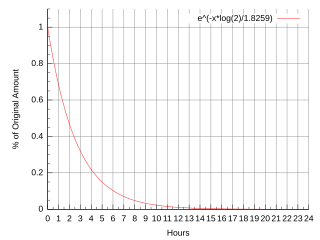
Fluorine-18 (18F) is a fluorine radioisotope which is an important source of positrons. It has a mass of 18.0009380(6) u and its half-life is 109.771(20) minutes. It decays by positron emission 96% of the time and electron capture 4% of the time. Both modes of decay yield stable oxygen-18.
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.
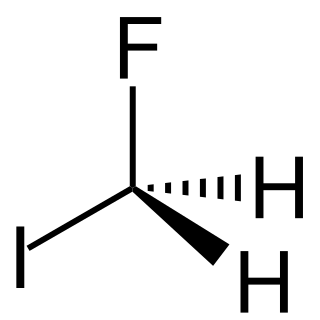
Fluoroiodomethane is the halomethane with the formula FCH2I. Also classified as a fluoroiodocarbon (FIC), it is a colorless liquid. It is a reagent for the introduction of the fluoromethyl (FCH2) group.

The Liebeskind–Srogl coupling reaction is an organic reaction forming a new carbon–carbon bond from a thioester and a boronic acid using a metal catalyst. It is a cross-coupling reaction. This reaction was invented by and named after Jiri Srogl from the Academy of Sciences, Czech Republic, and Lanny S. Liebeskind from Emory University, Atlanta, Georgia, USA. There are three generations of this reaction, with the first generation shown below. The original transformation used catalytic Pd(0), TFP = tris(2-furyl)phosphine as an additional ligand and stoichiometric CuTC = copper(I) thiophene-2-carboxylate as a co-metal catalyst. The overall reaction scheme is shown below.
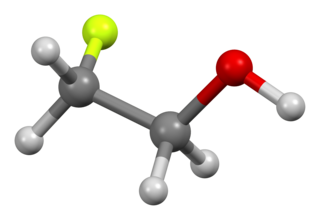
2-Fluoroethanol is the organic compound with the formula CH2FCH2OH. This colorless liquid is one of the simplest stable fluorinated alcohols. It was once used as a pesticide. The related difluoro- and trifluoroethanols are far less dangerous.

Brain positron emission tomography is a form of positron emission tomography (PET) that is used to measure brain metabolism and the distribution of exogenous radiolabeled chemical agents throughout the brain. PET measures emissions from radioactively labeled metabolically active chemicals that have been injected into the bloodstream. The emission data from brain PET are computer-processed to produce multi-dimensional images of the distribution of the chemicals throughout the brain.
Melanie Sarah Sanford is an American chemist, currently the Moses Gomberg Distinguished University Professor of Chemistry and Arthur F. Thurnau Professor of Chemistry at the University of Michigan. She is a Fellow for the American Association for the Advancement of Science, and was elected a member of the National Academy of Sciences and the American Academy of Arts and Sciences in 2016. She has served as an executive editor of the Journal of the American Chemical Society since 2021, having been an associate editor of the since 2014.
Radiofluorination is the process by which a radioactive isotope of fluorine is attached to a molecule and is preferably performed by nucleophilic substitution using nitro or halogens as leaving groups. Fluorine-18 is the most common isotope used for this procedure. This is due to its 97% positron emission and relatively long 109.8 min half-life. The half-life allows for a long enough time to be incorporated into the molecule and be used without causing exceedingly harmful effects. This process has many applications especially with the use of positron emission tomography (PET) as the aforementioned low positron energy is able to yield a high resolution in PET imaging.

Desmethoxyfallypride is a moderate affinity dopamine D2 receptor/D3 receptor antagonist used in medical research, usually in the form of the radiopharmaceuticals desmethoxyfallypride or DMFP(18F) and has been used in human studies as a positron emission tomography (PET) radiotracer.
Fluorodeoxyglycosylamine is a product of fluorodeoxyglucose and biological amines. The Maillard reaction of sugars and amines results in the formation of glycosylamines and Amadori products that are of biological significance, for drug delivery, role in central nervous system, and other potential applications.

18F-FMISO or fluoromisonidazole is a radiopharmaceutical used for PET imaging of hypoxia. It consists of a 2-nitroimidazole molecule labelled with the positron-emitter fluorine-18.

Dihydrotetrabenazine or DTBZ is an organic compound with the chemical formula C19H29NO3. It is a close analog of tetrabenazine. DTBZ and its derivatives, when labeled with positron emitting isotopes such as carbon-11 and fluorine-18, are used as PET radioligands for examining VMAT2.
Jason S. Lewis is a British radiochemist whose work relates to oncologic therapy and diagnosis. His research focus is a molecular imaging-based program focused on radiopharmaceutical development as well as the study of multimodality small- and biomolecule-based agents and their clinical translation. He has worked on the development of small molecules as well as radiolabeled peptides and antibodies probing the overexpression of receptors and antigens on tumors.
Neil Vasdev is a Canadian and American radiochemist and expert in nuclear medicine and molecular imaging, particularly in the application of PET. Radiotracers developed by the Vasdev Lab are in preclinical use worldwide, and many have been translated for first-in-human neuroimaging studies. He is the director and chief radiochemist of the Brain Health Imaging Centre and director of the Azrieli Centre for Neuro-Radiochemistry at the Centre for Addiction and Mental Health (CAMH). He is the Tier 1 Canada Research Chair in Radiochemistry and Nuclear Medicine, the endowed Azrieli Chair in Brain and Behaviour and Professor of Psychiatry at the University of Toronto. Vasdev has been featured on Global News, CTV, CNN, New York Times, Toronto Star and the Globe and Mail for his innovative research program.













