Insulin resistance (IR) is a pathological condition in which cells either fail to respond normally to the hormone insulin or downregulate insulin receptors in response to hyperinsulinemia.

Abdominal obesity, also known as central obesity and truncal obesity, is the human condition of an excessive concentration of visceral fat around the stomach and abdomen to such an extent that it is likely to harm its bearer's health. Abdominal obesity has been strongly linked to cardiovascular disease, Alzheimer's disease, and other metabolic and vascular diseases.

Leptin is a protein hormone predominantly made by adipocytes and its primary role is likely to regulate long-term energy balance.

Adipose tissue (also known as body fat, or simply fat) is a loose connective tissue composed mostly of adipocytes. In addition to adipocytes, adipose tissue contains the stromal vascular fraction(SVF) of cells including preadipocytes, fibroblasts, vascular endothelial cells and a variety of immune cells such as adipose tissue macrophages. Adipose tissue is derived from preadipocytes. Its main role is to store energy in the form of lipids, although it also cushions and insulates the body. Far from being hormonally inert, adipose tissue has, in recent years, been recognized as a major endocrine organ, as it produces hormones such as leptin, estrogen, resistin, and cytokines (especially TNFα). In obesity, adipose tissue is also implicated in the chronic release of pro-inflammatory markers known as adipokines, which are responsible for the development of metabolic syndrome, a constellation of diseases, including type 2 diabetes, cardiovascular disease and atherosclerosis. The two types of adipose tissue are white adipose tissue (WAT), which stores energy, and brown adipose tissue (BAT), which generates body heat. The formation of adipose tissue appears to be controlled in part by the adipose gene. Adipose tissue – more specifically brown adipose tissue – was first identified by the Swiss naturalist Conrad Gessner in 1551.

Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Adipocytes are derived from mesenchymal stem cells which give rise to adipocytes through adipogenesis. In cell culture, adipocyte progenitors can also form osteoblasts, myocytes and other cell types.
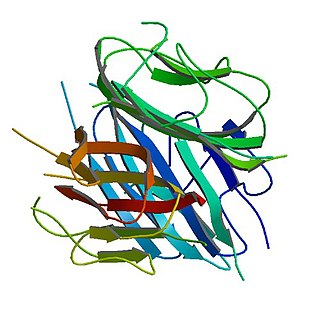
Adiponectin is a protein hormone and adipokine, which is involved in regulating glucose levels and fatty acid breakdown. In humans, it is encoded by the ADIPOQ gene and is produced primarily in adipose tissue, but also in muscle and even in the brain.
The adipokines, or adipocytokines are cytokines secreted by adipose tissue. Some contribute to an obesity-related low-grade state of inflammation or to the development of metabolic syndrome, a constellation of diseases including, but not limited to, type 2 diabetes, cardiovascular disease and atherosclerosis. The first adipokine to be discovered was leptin in 1994. Since that time, hundreds of adipokines have been discovered.

Hyperinsulinemia is a condition in which there are excess levels of insulin circulating in the blood relative to the level of glucose. While it is often mistaken for diabetes or hyperglycaemia, hyperinsulinemia can result from a variety of metabolic diseases and conditions, as well as non-nutritive sugars in the diet. While hyperinsulinemia is often seen in people with early stage type 2 diabetes mellitus, it is not the cause of the condition and is only one symptom of the disease. Type 1 diabetes only occurs when pancreatic beta-cell function is impaired. Hyperinsulinemia can be seen in a variety of conditions including diabetes mellitus type 2, in neonates and in drug-induced hyperinsulinemia. It can also occur in congenital hyperinsulinism, including nesidioblastosis.

Free Fatty acid receptor 4 (FFAR4), also termed G-protein coupled receptor 120 (GPR120), is a protein that in humans is encoded by the FFAR4 gene. This gene is located on the long arm of chromosome 10 at position 23.33. G protein-coupled receptors reside on their parent cells' surface membranes, bind any one of the specific set of ligands that they recognize, and thereby are activated to trigger certain responses in their parent cells. FFAR4 is a rhodopsin-like GPR in the broad family of GPRs which in humans are encoded by more than 800 different genes. It is also a member of a small family of structurally and functionally related GPRs that include at least three other free fatty acid receptors (FFARs) viz., FFAR1, FFAR2, and FFAR3. These four FFARs bind and thereby are activated by certain fatty acids.

alpha-2-HS-glycoprotein also known as fetuin-A is a protein that in humans is encoded by the AHSG gene. Fetuin-A belongs to the fetuin class of plasma binding proteins and is more abundant in fetal than adult blood.
Adipose tissue is an endocrine organ that secretes numerous protein hormones, including leptin, adiponectin, and resistin. These hormones generally influence energy metabolism, which is of great interest to the understanding and treatment of type 2 diabetes and obesity.

Chemerin, also known as retinoic acid receptor responder protein 2 (RARRES2), tazarotene-induced gene 2 protein (TIG2), or RAR-responsive protein TIG2 is a protein that in humans is encoded by the RARRES2 gene.
Most cases of type 2 diabetes involved many genes contributing small amount to the overall condition. As of 2011 more than 36 genes have been found that contribute to the risk of type 2 diabetes. All of these genes together still only account for 10% of the total genetic component of the disease.
A number of lifestyle factors are known to be important to the development of type 2 diabetes including: obesity, physical activity, diet, stress, and urbanization. Excess body fat underlies 64% of cases of diabetes in men and 77% of cases in women. A number of dietary factors such as sugar sweetened drinks and the type of fat in the diet appear to play a role.
Adipose tissue macrophages (ATMs) comprise tissue resident macrophages present in adipose tissue. Adipose tissue apart from adipocytes is composed of the stromal vascular fraction (SVF) of cells including preadipocytes, fibroblasts, vascular endothelial cells and variety of immune cells. The latter ones are composed of mast cells, eosinophils, B cells, T cells and macrophages. The number of macrophages within adipose tissue differs depending on the metabolic status. As discovered by Rudolph Leibel and Anthony Ferrante et al. in 2003 at Columbia University, the percentage of macrophages within adipose tissue ranges from 10% in lean mice and humans up to 50% in extremely obese, leptin deficient mice and almost 40% in obese humans. Increased number of adipose tissue macrophages correlates with increased adipose tissue production of proinflammatory molecules and might therefore contribute to the pathophysiological consequences of obesity.

Christos Socrates Mantzoros is a Greek American physician-scientist, practicing internist-endocrinologist, teacher and researcher. He is a professor of medicine at Harvard Medical School and an adjunct professor at Boston University School of Medicine. He currently serves as the chief of endocrinology, diabetes and metabolism at the VA Boston Healthcare System, where he created de novo a leading academic division true to its tripartite mission and as the founding director of human nutrition at Beth Israel Deaconess Medical Center (BIDMC), Harvard Medical School. Finally, he holds the editor-in-chief position of the journal Metabolism: Clinical and Experimental.

Pathophysiology of obesity is the study of disordered physiological processes that cause, result from, or are otherwise associated with obesity. A number of possible pathophysiological mechanisms have been identified which may contribute in the development and maintenance of obesity.
Diabetes mellitus (DM) is a type of metabolic disease characterized by hyperglycemia. It is caused by either defected insulin secretion or damaged biological function, or both. The high-level blood glucose for a long time will lead to dysfunction of a variety of tissues.
Meteorin-like/Meteorin-Beta (Metrnl)/IL-41, also known as subfatin and cometin, is a small (~27kDa) secreted cytokine, protein encoded by a gene called meteorin-like (METRNL). METRNL, also known as subfatin and cometin, is highly expressed in mucosal tissues, skin and activated macrophages. Metrnl has also been described to be a hormone

Serpin A12 is a glycoprotein that is a class A member of the serine protease inhibitor (serpin) family. In humans, Serpin A12 is encoded by the SERPINA12 gene.
















