Related Research Articles

Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine.

Lactose, or milk sugar, is a disaccharide sugar synthesized by galactose and glucose subunits and has the molecular formula C12H22O11. Lactose makes up around 2–8% of milk (by mass). The name comes from lac (gen. lactis), the Latin word for milk, plus the suffix -ose used to name sugars. The compound is a white, water-soluble, non-hygroscopic solid with a mildly sweet taste. It is used in the food industry.

Carl Wilhelm Scheele was a Swedish German pharmaceutical chemist.

Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. It is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E334 and to impart its distinctive sour taste. Naturally occurring tartaric acid is a useful raw material in organic chemical synthesis. Tartaric acid, an alpha-hydroxy-carboxylic acid, is diprotic and aldaric in acid characteristics, and is a dihydroxyl derivative of succinic acid.

Jacques Necker was a Genevan banker and statesman who served as finance minister for Louis XVI. He was a reformer, but his innovations sometimes caused great discontent. Necker was a constitutional monarchist, a political economist, and a moralist, who wrote a severe critique of the new principle of equality before the law.

The Marsh test is a highly sensitive method in the detection of arsenic, especially useful in the field of forensic toxicology when arsenic was used as a poison. It was developed by the chemist James Marsh and first published in 1836. The method continued to be used, with improvements, in forensic toxicology until the 1970s.

Arsine (IUPAC name: arsane) is an inorganic compound with the formula AsH3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in the semiconductor industry and for the synthesis of organoarsenic compounds. The term arsine is commonly used to describe a class of organoarsenic compounds of the formula AsH3−xRx, where R = aryl or alkyl. For example, As(C6H5)3, called triphenylarsine, is referred to as "an arsine".
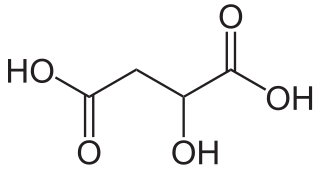
Malic acid is an organic compound with the molecular formula C4H6O5. It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms, though only the L-isomer exists naturally. The salts and esters of malic acid are known as malates. The malate anion is an intermediate in the citric acid cycle.

Gallic acid (also known as 3,4,5-trihydroxybenzoic acid) is a trihydroxybenzoic acid with the formula C6H2(OH)3CO2H. It is classified as a phenolic acid. It is found in gallnuts, sumac, witch hazel, tea leaves, oak bark, and other plants. It is a white solid, although samples are typically brown owing to partial oxidation. Salts and esters of gallic acid are termed "gallates".
The year 1784 in science and technology involved some significant events.

Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula HO2C−CO2H, also written as (CO2H)2. It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus Oxalis, commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous.
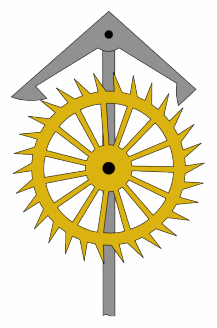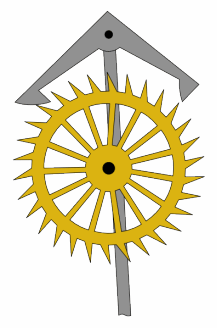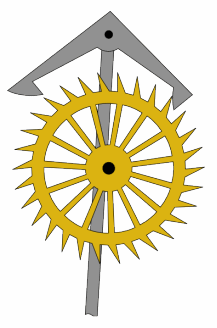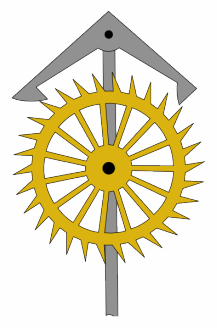
An escapement is a mechanical linkage in mechanical watches and clocks that gives impulses to the timekeeping element and periodically releases the gear train to move forward, advancing the clock's hands. The impulse action transfers energy to the clock's timekeeping element to replace the energy lost to friction during its cycle and keep the timekeeper oscillating. The escapement is driven by force from a coiled spring or a suspended weight, transmitted through the timepiece's gear train. Each swing of the pendulum or balance wheel releases a tooth of the escapement's escape wheel, allowing the clock's gear train to advance or "escape" by a fixed amount. This regular periodic advancement moves the clock's hands forward at a steady rate. At the same time, the tooth gives the timekeeping element a push, before another tooth catches on the escapement's pallet, returning the escapement to its "locked" state. The sudden stopping of the escapement's tooth is what generates the characteristic "ticking" sound heard in operating mechanical clocks and watches.

Boron sulfide is the chemical compound with the formula B2S3. It is a white, moisture-sensitive solid. It has a polymeric structure. The material has been of interest as a component of "high-tech" glasses and as a reagent for preparing organosulfur compounds.

The Peltigeraceae are a family of lichens in the order Peltigerales. The Peltigeraceae, which contains 15 genera and about 600 species, has recently (2018) been emended to include the families Lobariaceae and Nephromataceae. Many Peltigeraceae species have large and conspicuous, leathery thalli. They largely occur in cool-temperate to tropical montane climates. Tripartite thalli involving fungus, green algae and cyanobacteria are common in this family.

Wagneria is a genus of flies in the family Tachinidae. More junior homonyms exist of Wagneria than any other animal genus name.
Litophasia is a genus of flies in the family Tachinidae.

Homopus areolatus, commonly known as the common padloper or parrot-beaked tortoise, is a tiny species of tortoise of the genus Homopus, indigenous to the southern part of South Africa.

Phasia obesa is a species of 'parasitic flies' belonging to the family Tachinidae subfamily Phasiinae.
Robert Porrett (1783–1868) was an English amateur chemist and antiquary.

Fluorine is a relatively new element in human applications. In ancient times, only minor uses of fluorine-containing minerals existed. The industrial use of fluorite, fluorine's source mineral, was first described by early scientist Georgius Agricola in the 16th century, in the context of smelting. The name "fluorite" derives from Agricola's invented Latin terminology. In the late 18th century, hydrofluoric acid was discovered. By the early 19th century, it was recognized that fluorine was a bound element within compounds, similar to chlorine. Fluorite was determined to be calcium fluoride.
References
- ↑ Scheele, Carl Wilhelm (1780). "Om Mjölk och dess syra" (About milk and its acid), Kongliga Vetenskaps Academiens Nya Handlingar (New Proceedings of the Royal Academy of Science), 1 : 116-124. From page 116: "Det år bekant, at Ko-mjölk innehåller Smör, Ost, Mjölk-såcker, … " (It is known, that cow's milk contains butter, cheese, milk-sugar, … ); "Om Mjölk-Såcker-Syra" (On milk-sugar acid), Kongliga Vetenskaps Academiens Nya Handlingar (New Proceedings of the Royal Academy of Science), 1 : 269-275. From pages 269–270: "Mjölk-Såcker år et sal essentiale, som uti Mjölken finnes uplöst, och som, för dess sötaktiga smak skull, fått namn af såcker." (Milk sugar is an essential salt, which is found dissolved in milk, and which, on account of its sweet taste, has the name of "sugar".)
- ↑ Wolfe, John J. (1999). Brandy, Balloons, & Lamps: Ami Argand, 1750-1803. Carbondale: Southern Illinois University Press. ISBN 0-8093-2278-1.
- ↑ "Earnshaw's Chronometer Escapement". Antique Pocket Watches on the Internet. London: Pieces of Time. Archived from the original on 2012-02-04. Retrieved 2012-03-09.
- ↑ "Copley Medal | British scientific award". Encyclopedia Britannica. Retrieved 21 July 2020.
- ↑ "Abel, Clarke (1789-1826)". catalogue.bnf.fr (in French). Bibliothèque Nationale de France. Retrieved 6 February 2021.