In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.

Acetoacetic acid is the organic compound with the formula CH3COCH2COOH. It is the simplest beta-keto acid, and like other members of this class, it is unstable. The methyl and ethyl esters, which are quite stable, are produced on a large scale industrially as precursors to dyes. Acetoacetic acid is a weak acid.

In organic chemistry, a dicarbonyl is a molecule containing two carbonyl groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical or unsymmetrical.

In organic chemistry, alkenols are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond. The terms enol and alkenol are portmanteaus deriving from "-ene"/"alkene" and the "-ol" suffix indicating the hydroxyl group of alcohols, dropping the terminal "-e" of the first term. Generation of enols often involves deprotonation at the α position to the carbonyl group—i.e., removal of the hydrogen atom there as a proton H+. When this proton is not returned at the end of the stepwise process, the result is an anion termed an enolate. The enolate structures shown are schematic; a more modern representation considers the molecular orbitals that are formed and occupied by electrons in the enolate. Similarly, generation of the enol often is accompanied by "trapping" or masking of the hydroxy group as an ether, such as a silyl enol ether.

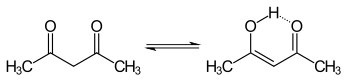
Tautomers are structural isomers of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life.

Acetone is an organic compound with the formula (CH3)2CO. It is the simplest and smallest ketone. It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor.

Meldrum's acid or 2,2-dimethyl-1,3-dioxane-4,6-dione is an organic compound with formula C6H8O4. Its molecule has a heterocyclic core with four carbon and two oxygen atoms; the formula can also be written as [−O−(C 2)−O−(C=O)−(CH2)−(C=O)−].
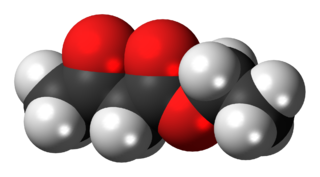
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is a colorless liquid. It is widely used as a chemical intermediate in the production of a wide variety of compounds. It is used as a flavoring for food.

Benzylideneacetone is the organic compound described by the formula C6H5CH=CHC(O)CH3. Although both cis- and trans-isomers are possible for the α,β-unsaturated ketone, only the trans isomer is observed. Its original preparation demonstrated the scope of condensation reactions to construct new, complex organic compounds. Benzylideneacetone is used as a flavouring ingredient in food and perfumes.

Nickel(II) bis(acetylacetonate) is a coordination complex with the formula [Ni(acac)2]3, where acac is the anion C5H7O2− derived from deprotonation of acetylacetone. It is a dark green paramagnetic solid that is soluble in organic solvents such as toluene. It reacts with water to give the blue-green diaquo complex Ni(acac)2(H2O)2.

Ruthenium(III) acetylacetonate is a coordination complex with the formula Ru(O2C5H7)3. O2C5H7− is the ligand called acetylacetonate. This compound exists as a dark violet solid that is soluble in most organic solvents. It is used as a precursor to other compounds of ruthenium.
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion (CH
3COCHCOCH−
3) and metal ions, usually transition metals. The bidentate ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl (RCOCHCOR′−). Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis, and precursors to industrial hydroformylation catalysts. C
5H
7O−
2 in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).

Vanadyl acetylacetonate is the chemical compound with the formula VO(acac)2, where acac– is the conjugate base of acetylacetone. It is a blue-green solid that dissolves in polar organic solvents. The coordination complex consists of the vanadyl group, VO2+, bound to two acac– ligands via the two oxygen atoms on each. Like other charge-neutral acetylacetonate complexes, it is not soluble in water.

Dibenzoylmethane (DBM) is an organic compound with the formula (C6H5C(O))2CH2. DBM is the name for a 1,3-diketone, but the compound exists primarily as one of two equivalent enol tautomers. DBM (actually its enol) is a white solid. Due to their high photostability and UV-absorbing properties, derivatives of DBM such as avobenzone, have found applications as sunscreen products.
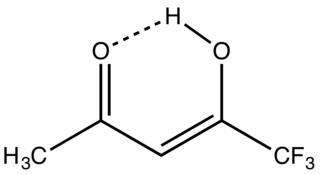
1,1,1-Trifluoroacetylacetone is the organofluorine compound with the formula CF3C(O)CH2C(O)CH3. It is a colorless liquid. Like other 1,3-diketones, it is used as a precursor to heterocycles, e.g. pyrazoles, and metal chelates. It is prepared by condensation of esters of trifluoroacetic acid with acetone.

3,5-Dimethylpyrazole is an organic compound with the formula (CH3C)2CHN2H. It is one of several isomeric derivatives of pyrazole that contain two methyl substituents. The compound is unsymmetrical but the corresponding conjugate acid (pyrazolium) and conjugate base (pyrazolide) have C2v symmetry. It is a white solid that dissolves well in polar organic solvents.

Tetraacetylethane is the organic compound with the nominal formula [CH(C(O)CH3)2]2. It is a white solid that has attracted interest as a precursor to heterocycles and metal complexes. It is prepared by oxidation of sodium acetylacetonate:

Sodium acetylacetonate is an organic compound with the nominal formula Na[CH(C(O)CH3)2]. This white, water-soluble solid is the conjugate base of acetylacetone.
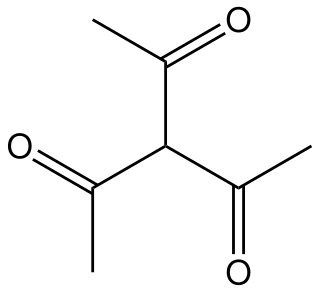
Triacetylmethane is the organic compound with the formula HC(C CH3)3. It is a colorless liquid that is soluble in organic solvents and in alkaline water. It readily forms an enolate. The enolate forms a variety of metal complexes related to the metal acetylacetonates.