
Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives have been used for centuries as part of an effort to preserve food, for example vinegar (pickling), salt (salting), smoke (smoking), sugar (crystallization), etc. This allows for longer-lasting foods such as bacon, sweets or wines. With the advent of ultra-processed foods in the second half of the twentieth century, many additives have been introduced, of both natural and artificial origin. Food additives also include substances that may be introduced to food indirectly in the manufacturing process, through packaging, or during storage or transport.
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are relatively stronger acids. Alcohols, with –OH, can act as acids but they are usually very weak. The relative stability of the conjugate base of the acid determines its acidity. Other groups can also confer acidity, usually weakly: the thiol group –SH, the enol group, and the phenol group. In biological systems, organic compounds containing these groups are generally referred to as organic acids.

Trisodium phosphate (TSP) is an inorganic compound with the chemical formula Na3PO4. It is a white, granular or crystalline solid, highly soluble in water, producing an alkaline solution. TSP is used as a cleaning agent, builder, lubricant, food additive, stain remover, and degreaser.

Trisodium citrate has the molecular formula Na3C6H5O7. It is sometimes referred to simply as "sodium citrate", though sodium citrate can refer to any of the three sodium salts of citric acid. It possesses a saline, mildly tart flavor, and is a mild alkali.
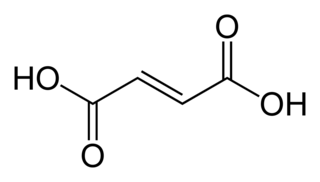
Fumaric acid is an organic compound with the formula HO2CCH=CHCO2H. A white solid, fumaric acid occurs widely in nature. It has a fruit-like taste and has been used as a food additive. Its E number is E297. The salts and esters are known as fumarates. Fumarate can also refer to the C
4H
2O2−
4 ion (in solution). Fumaric acid is the trans isomer of butenedioic acid, while maleic acid is the cis isomer.

Sodium benzoate also known as benzoate of soda is the sodium salt of benzoic acid, widely used as a food preservative (with an E number of E211) and a pickling agent. It appears as a white crystalline chemical with the formula C6H5COONa.

Adipic acid or hexanedioic acid is the organic compound with the formula (CH2)4(COOH)2. From an industrial perspective, it is the most important dicarboxylic acid: about 2.5 billion kilograms of this white crystalline powder are produced annually, mainly as a precursor for the production of nylon. Adipic acid otherwise rarely occurs in nature, but it is known as manufactured E number food additive E355. Salts and esters of adipic acid are known as adipates.

Sodium acetate, CH3COONa, also abbreviated NaOAc, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.

Thioglycolic acid (TGA) is the organic compound HSCH2CO2H. TGA is often called mercaptoacetic acid (MAA). It contains both a thiol (mercaptan) and carboxylic acid functional groups. It is a colorless liquid with a strongly unpleasant odor. TGA is miscible with polar organic solvents.

A tartrate is a salt or ester of the organic compound tartaric acid, a dicarboxylic acid. The formula of the tartrate dianion is O−OC-CH(OH)-CH(OH)-COO− or C4H4O62−.
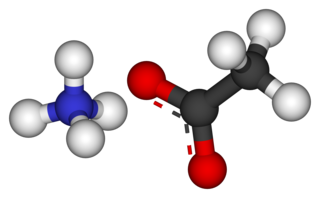
Ammonium acetate, also known as spirit of Mindererus in aqueous solution, is a chemical compound with the formula NH4CH3CO2. It is a white, hygroscopic solid and can be derived from the reaction of ammonia and acetic acid. It is available commercially.

Sodium ascorbate is one of a number of mineral salts of ascorbic acid (vitamin C). The molecular formula of this chemical compound is C6H7NaO6. As the sodium salt of ascorbic acid, it is known as a mineral ascorbate. It has not been demonstrated to be more bioavailable than any other form of vitamin C supplement.

Potassium acetate (also called potassium ethanoate), (CH3COOK) is the potassium salt of acetic acid. It is a hygroscopic solid at room temperature.

Caramel color or caramel coloring is a water-soluble food coloring. It is made by heat treatment of carbohydrates (sugars), in general in the presence of acids, alkalis, or salts, in a process called caramelization. It is more fully oxidized than caramel candy, and has an odor of burnt sugar and a somewhat bitter taste. Its color ranges from pale yellow to amber to dark brown.
Sodium adipate is a chemical organic compound with formula Na2C6H8O4. It is the sodium salt of adipic acid.

Sodium malate is a compound with formula Na2(C2H4O(COO)2). It is the sodium salt of malic acid. As a food additive, it has the E number E350.
Acidulants are chemical compounds that give a tart, sour, or acidic flavor to foods or enhance the perceived sweetness of foods. Acidulants can also function as leavening agents and emulsifiers in some kinds of processed foods. Though acidulants can lower pH they differ from acidity regulators, which are food additives specifically intended to modify the stability of food or enzymes within it. Typical acidulants are acetic acid and citric acid. Many beverages, such as colas, contain phosphoric acid. Sour candies often are formulated with malic acid. Other acidulants used in food production include: fumaric acid, tartaric acid, lactic acid and gluconic acid.
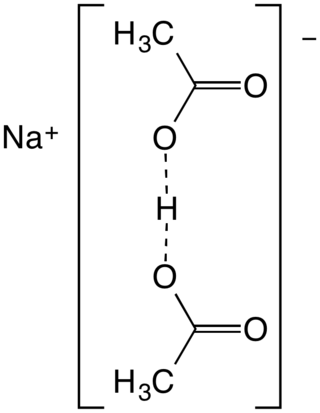
Sodium diacetate is a compound with formula NaH(C
2H
3O
2)
2. It is a salt of acetic acid. It is a colorless solid that is used in seasonings and as an antimicrobial agent.

Wine preservatives are used to preserve the quality and shelf life of bottled wine without affecting its taste. Specifically, they are used to prevent oxidation and bacterial spoilage by inhibiting microbial activity.

















