Related Research Articles

Refrigeration is any of various types of cooling of a space, substance, or system to lower and/or maintain its temperature below the ambient one. Refrigeration is an artificial, or human-made, cooling method.

A heat pump is a device that uses work to transfer heat from a cool space to a warm space by transferring thermal energy using a refrigeration cycle, cooling the cool space and warming the warm space. In cold weather, a heat pump can move heat from the cool outdoors to warm a house; the pump may also be designed to move heat from the house to the warmer outdoors in warm weather. As they transfer heat rather than generating heat, they are more energy-efficient than other ways of heating or cooling a home.

Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid is dissolved by or permeates a liquid or solid. While adsorption does often precede absorption, which involves the transfer of the absorbate into the volume of the absorbent material, alternatively, adsorption is distinctly a surface phenomenon, wherein the adsorbate does not penetrate through the material surface and into the bulk of the adsorbent. The term sorption encompasses both adsorption and absorption, and desorption is the reverse of sorption.
For fluid power, a working fluid is a gas or liquid that primarily transfers force, motion, or mechanical energy. In hydraulics, water or hydraulic fluid transfers force between hydraulic components such as hydraulic pumps, hydraulic cylinders, and hydraulic motors that are assembled into hydraulic machinery, hydraulic drive systems, etc. In pneumatics, the working fluid is air or another gas which transfers force between pneumatic components such as compressors, vacuum pumps, pneumatic cylinders, and pneumatic motors. In pneumatic systems, the working gas also stores energy because it is compressible.

A chiller is a machine that removes heat from a liquid coolant via a vapor-compression, absorption refrigeration, or absorption refrigeration cycles. This liquid can then be circulated through a heat exchanger to cool equipment, or another process stream. As a necessary by-product, refrigeration creates waste heat that must be exhausted to ambience, or for greater efficiency, recovered for heating purposes. Vapor compression chillers may use any of a number of different types of compressors. Most common today are the hermetic scroll, semi-hermetic screw, or centrifugal compressors. The condensing side of the chiller can be either air or water cooled. Even when liquid cooled, the chiller is often cooled by an induced or forced draft cooling tower. Absorption and adsorption chillers require a heat source to function.
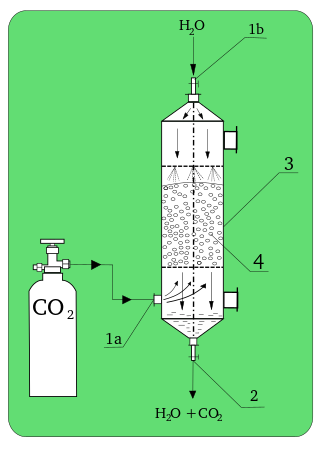
In chemistry, absorption is a physical or chemical phenomenon or a process in which atoms, molecules or ions enter some bulk phase – liquid or solid material. This is a different process from adsorption, since molecules undergoing absorption are taken up by the volume, not by the surface.

An absorption refrigerator is a refrigerator that uses a heat source to provide the energy needed to drive the cooling process. Solar energy, burning a fossil fuel, waste heat from factories, and district heating systems are examples of convenient heat sources that can be used. An absorption refrigerator uses two coolants: the first coolant performs evaporative cooling and then is absorbed into the second coolant; heat is needed to reset the two coolants to their initial states. Absorption refrigerators are commonly used in recreational vehicles (RVs), campers, and caravans because the heat required to power them can be provided by a propane fuel burner, by a low-voltage DC electric heater or by a mains-powered electric heater. Absorption refrigerators can also be used to air-condition buildings using the waste heat from a gas turbine or water heater in the building. Using waste heat from a gas turbine makes the turbine very efficient because it first produces electricity, then hot water, and finally, air-conditioning—trigeneration.
A zeotropicmixture, or non-azeotropic mixture, is a mixture with liquid components that have different boiling points. For example, nitrogen, methane, ethane, propane, and isobutane constitute a zeotropic mixture. Individual substances within the mixture do not evaporate or condense at the same temperature as one substance. In other words, the mixture has a temperature glide, as the phase change occurs in a temperature range of about four to seven degrees Celsius, rather than at a constant temperature. On temperature-composition graphs, this temperature glide can be seen as the temperature difference between the bubble point and dew point. For zeotropic mixtures, the temperatures on the bubble (boiling) curve are between the individual component's boiling temperatures. When a zeotropic mixture is boiled or condensed, the composition of the liquid and the vapor changes according to the mixtures's temperature-composition diagram.

Vapour-compression refrigeration or vapor-compression refrigeration system (VCRS), in which the refrigerant undergoes phase changes, is one of the many refrigeration cycles and is the most widely used method for air conditioning of buildings and automobiles. It is also used in domestic and commercial refrigerators, large-scale warehouses for chilled or frozen storage of foods and meats, refrigerated trucks and railroad cars, and a host of other commercial and industrial services. Oil refineries, petrochemical and chemical processing plants, and natural gas processing plants are among the many types of industrial plants that often utilize large vapor-compression refrigeration systems. Cascade refrigeration systems may also be implemented using two compressors.
Solar air conditioning, or "solar-powered air conditioning", refers to any air conditioning (cooling) system that uses solar power.

An absorption heat pump (AHP) is a heat pump driven by thermal energy such as combustion of natural gas, steam solar-heated water, air or geothermal-heated water differently from compression heat pumps that are driven by mechanical energy. AHPs are more complex and require larger units compared to compression heat pumps. In particular, the lower electricity demand of such heat pumps is related to the liquid pumping only. Their applications are restricted to those cases when electricity is extremely expensive or a large amount of unutilized heat at suitable temperatures is available and when the cooling or heating output has a greater value than heat input consumed. Absorption refrigerators also work on the same principle, but are not reversible and cannot serve as a heat source.

An air source heat pump (ASHP) is a heat pump that can absorb heat from air outside a building and release it inside; it uses the same vapor-compression refrigeration process and much the same equipment as an air conditioner, but in the opposite direction. ASHPs are the most common type of heat pump and, usually being smaller, tend to be used to heat individual houses or flats rather than blocks, districts or industrial processes.

A transcritical cycle is a closed thermodynamic cycle where the working fluid goes through both subcritical and supercritical states. In particular, for power cycles the working fluid is kept in the liquid region during the compression phase and in vapour and/or supercritical conditions during the expansion phase. The ultrasupercritical steam Rankine cycle represents a widespread transcritical cycle in the electricity generation field from fossil fuels, where water is used as working fluid. Other typical applications of transcritical cycles to the purpose of power generation are represented by organic Rankine cycles, which are especially suitable to exploit low temperature heat sources, such as geothermal energy, heat recovery applications or waste to energy plants. With respect to subcritical cycles, the transcritical cycle exploits by definition higher pressure ratios, a feature that ultimately yields higher efficiencies for the majority of the working fluids. Considering then also supercritical cycles as a valid alternative to the transcritical ones, the latter cycles are capable of achieving higher specific works due to the limited relative importance of the work of compression work. This evidences the extreme potential of transcritical cycles to the purpose of producing the most power with the least expenditure.

Thermodynamic heat pump cycles or refrigeration cycles are the conceptual and mathematical models for heat pump, air conditioning and refrigeration systems. A heat pump is a mechanical system that transmits heat from one location at a certain temperature to another location at a higher temperature. Thus a heat pump may be thought of as a "heater" if the objective is to warm the heat sink, or a "refrigerator" or “cooler” if the objective is to cool the heat source. The operating principles in both cases are the same; energy is used to move heat from a colder place to a warmer place.
Natural refrigerants are considered substances that serve as refrigerants in refrigeration systems. They are alternatives to synthetic refrigerants such as chlorofluorocarbon (CFC), hydrochlorofluorocarbon (HCFC), and hydrofluorocarbon (HFC) based refrigerants. Unlike other refrigerants, natural refrigerants can be found in nature and are commercially available thanks to physical industrial processes like fractional distillation, chemical reactions such as Haber process and spin-off gases. The most prominent of these include various natural hydrocarbons, carbon dioxide, ammonia, and water. Natural refrigerants are preferred actually in new equipment to their synthetic counterparts for their presumption of higher degrees of sustainability. With the current technologies available, almost 75 percent of the refrigeration and air conditioning sector has the potential to be converted to natural refrigerants.
The term subcooling refers to a liquid existing at a temperature below its normal boiling point. For example, water boils at 373 K; at room temperature (293 K) liquid water is termed "subcooled". A subcooled liquid is the convenient state in which, say, refrigerants may undergo the remaining stages of a refrigeration cycle. Normally, a refrigeration system has a subcooling stage, allowing technicians to be certain that the quality, in which the refrigerant reaches the next step on the cycle, is the desired one. Subcooling may take place in heat exchangers and outside them. Being both similar and inverse processes, subcooling and superheating are important to determine stability and well-functioning of a refrigeration system.
Professor Robert Critoph is a British academic in the field of mechanical engineering, working on heating and cooling technologies and energy demand. Critoph is Director of the Interdisciplinary Centre for Storage, Transformation and Upgrading of Thermal Energy (i-STUTE). I-STUTE is one of six End Use Energy Demand Centres.
Heat engines, refrigeration cycles and heat pumps usually involve a fluid to and from which heat is transferred while undergoing a thermodynamic cycle. This fluid is called the working fluid. Refrigeration and heat pump technologies often refer to working fluids as refrigerants. Most thermodynamic cycles make use of the latent heat of the working fluid. In case of other cycles the working fluid remains in gaseous phase while undergoing all the processes of the cycle. When it comes to heat engines, working fluid generally undergoes a combustion process as well, for example in internal combustion engines or gas turbines. There are also technologies in heat pump and refrigeration, where working fluid does not change phase, such as reverse Brayton or Stirling cycle.
Sorption enhanced water gas shift (SEWGS) is a technology that combines a pre-combustion carbon capture process with the water gas shift reaction (WGS) in order to produce a hydrogen rich stream from the syngas fed to the SEWGS reactor.
The ionocaloric refrigeration cycle is an advanced cooling technology that utilizes the ionocaloric effect, driven by an electrochemical field, to achieve efficient and eco-friendly refrigeration. By manipulating the electrochemical potential through ion addition or removal, significant temperature changes and entropy variations are achieved. This cycle offers a sustainable alternative to traditional refrigeration systems, with potential applications in various industries. Ongoing research is focused on optimizing ionocaloric materials and system design to enhance its performance and viability.
References
- 1 2 3 4 5 R.E. Critoph, R.E. (2007). "Adsorption Refrigeration Research at Warwick" (PDF). warwick.ac.uk. Archived (PDF) from the original on 2021-01-16. Retrieved 2020-05-31.
- 1 2 Vasiliev, L. L; Mishkinis, D. A; Antukh, A. A; Vasiliev, L. L (2001-04-01). "Solar–gas solid sorption heat pump". Applied Thermal Engineering. 21 (5): 573–583. doi:10.1016/S1359-4311(00)00069-7. ISSN 1359-4311.
- ↑ Hawaii Energy and Environmental Technologies (HEET) Initiative.
- 1 2 Rupam, Tahmid Hasan; Islam, Md. Amirul; Pal, Animesh; Saha, Bidyut Baran (2020-07-05). "Adsorption thermodynamics and performance indicators of selective adsorbent/refrigerant pairs". Applied Thermal Engineering. 175: 115361. doi:10.1016/j.applthermaleng.2020.115361. ISSN 1359-4311. S2CID 218777958.
- ↑ Goyal, Parash; Baredar, Prashant; Mittal, Arvind; Siddiqui, Ameenur. R. (2016-01-01). "Adsorption refrigeration technology – An overview of theory and its solar energy applications". Renewable and Sustainable Energy Reviews. 53: 1389–1410. doi:10.1016/j.rser.2015.09.027. ISSN 1364-0321.
- ↑ "Gas driven heat pumps" (PDF). London: Department for Business, Energy & Industrial Strategy. September 2016.