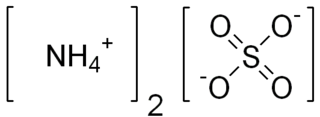Ammonia is a compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. It is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45 percent of the world's food and fertilizers. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water.
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.

Ammonium perchlorate ("AP") is an inorganic compound with the formula NH4ClO4. It is a colorless or white solid that is soluble in water. It is a powerful oxidizer. Combined with a fuel, it can be used as a rocket propellant. Its instability has involved it in a number of accidents, such as the PEPCON disaster.
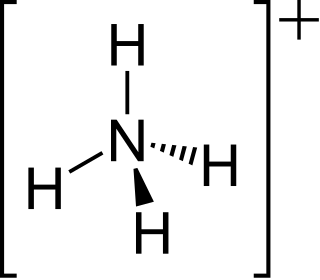
The ammonium cation is a positively charged polyatomic ion with the chemical formula NH+
4. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic groups.

Ammonium nitrate is a chemical compound with the chemical formula NH4NO3. It is a white crystalline solid consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer. Global production was estimated at 21.6 million tonnes in 2017.

Ammonium chloride is an inorganic compound with the formula NH4Cl and a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. In its naturally occurring mineralogic form, it is known as sal ammoniac. The mineral is commonly formed on burning coal dumps from condensation of coal-derived gases. It is also found around some types of volcanic vents. It is mainly used as fertilizer and a flavouring agent in some types of liquorice. It is the product from the reaction of hydrochloric acid and ammonia.

Ammonium bicarbonate is an inorganic compound with formula (NH4)HCO3, simplified to NH5CO3. The compound has many names, reflecting its long history. Chemically speaking, it is the bicarbonate salt of the ammonium ion. It is a colourless solid that degrades readily to carbon dioxide, water and ammonia.

Ammonium carbonate is a salt with the chemical formula (NH4)2CO3. Since it readily degrades to gaseous ammonia and carbon dioxide upon heating, it is used as a leavening agent and also as smelling salt. It is also known as baker's ammonia and was a predecessor to the more modern leavening agents baking soda and baking powder. It is a component of what was formerly known as sal volatile and salt of hartshorn, and produces a pungent smell when baked.

Quaternary ammonium cations, also known as quats, are positively charged polyatomic ions of the structure NR+
4, R being an alkyl group or an aryl group. Unlike the ammonium ion and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule.
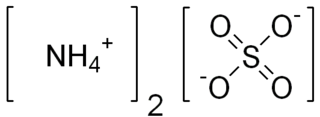
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.

Ammonium fluoride is the inorganic compound with the formula NH4F. It crystallizes as small colourless prisms, having a sharp saline taste, and is highly soluble in water. Like all fluoride salts, it is moderately toxic in both acute and chronic overdose.

Ammonium nitrite, NH4NO2, is the ammonium salt of nitrous acid. It is not used in pure isolated form since it is highly unstable and decomposes into water and nitrogen, even at room temperature.

Ammonium hydrosulfide is the chemical compound with the formula (NH4)HS.

Ammonium uranyl carbonate (UO2CO3·2(NH4)2CO3) is known in the uranium processing industry as AUC and is also called uranyl ammonium carbonate. This compound is important as a component in the conversion process of uranium hexafluoride (UF6) to uranium dioxide (UO2). The ammonium uranyl carbonate is combined with steam and hydrogen at 500–600 °C to yield UO2. In another process aqueous uranyl nitrate, known as uranyl nitrate liquor (UNL) is treated with ammonium bicarbonate to form ammonium uranyl carbonate as a solid precipitate. This is separated from the solution, dried with methanol and then calcinated with hydrogen directly to UO2 to obtain a sinterable grade powder. The ex-AUC uranium dioxide powder is free-flowing, relatively coarse (10 µ) and porous with specific surface area in the range of 5 m2/g and suitable for direct pelletisation, avoiding the granulation step. Conversion to UO2 is often performed as the first stage of nuclear fuel fabrication.

Ammonium hydrogen fluoride is the inorganic compound with the formula NH4HF2 or NH4F·HF. It is produced from ammonia and hydrogen fluoride. This colourless salt is a glass-etchant and an intermediate in a once-contemplated route to hydrofluoric acid.
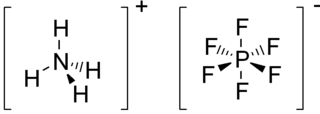
Ammonium hexafluorophosphate is the inorganic compound with the formula NH4PF6. It is a white water-soluble, hygroscopic solid. The compound is a salt consisting of the ammonium cation and hexafluorophosphate anion. It is commonly used as a source of the hexafluorophosphate anion, a weakly coordinating anion. It is prepared by combining neat ammonium fluoride and phosphorus pentachloride. Alternatively it can also be produced from phosphonitrilic chloride:

Ammonium permanganate is the chemical compound NH4MnO4, or NH3·HMnO4. It is a water soluble, violet-brown or dark purple salt.

Ammonium fluorosilicate (also known as ammonium hexafluorosilicate, ammonium fluosilicate or ammonium silicofluoride) has the formula (NH4)2SiF6. It is a toxic chemical, like all salts of fluorosilicic acid. It is made of white crystals, which have at least three polymorphs and appears in nature as rare minerals cryptohalite or bararite.

Ammonium sulfite is the ammonium salt of sulfurous acid with the chemical formula (NH4)2SO3.
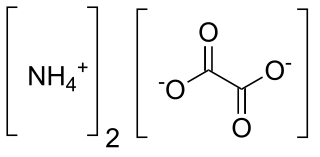
Ammonium oxalate, C2H8N2O4 – more commonly written as (NH4)2C2O4 – is an oxalate salt with ammonium (sometimes as a monohydrate). It is a colorless (white) salt under standard conditions and is odorless and non-volatile. It is the ammonium salt of oxalic acid, and occurs in many plants and vegetables.