Cell biology is a branch of biology that studies the structure, function, and behavior of cells. All living organisms are made of cells. A cell is the basic unit of life that is responsible for the living and functioning of organisms. Cell biology is the study of the structural and functional units of cells. Cell biology encompasses both prokaryotic and eukaryotic cells and has many subtopics which may include the study of cell metabolism, cell communication, cell cycle, biochemistry, and cell composition. The study of cells is performed using several microscopy techniques, cell culture, and cell fractionation. These have allowed for and are currently being used for discoveries and research pertaining to how cells function, ultimately giving insight into understanding larger organisms. Knowing the components of cells and how cells work is fundamental to all biological sciences while also being essential for research in biomedical fields such as cancer, and other diseases. Research in cell biology is interconnected to other fields such as genetics, molecular genetics, molecular biology, medical microbiology, immunology, and cytochemistry.

A lysosome is a membrane-bound organelle found in many animal cells. They are spherical vesicles that contain hydrolytic enzymes that can break down many kinds of biomolecules. A lysosome has a specific composition, of both its membrane proteins, and its lumenal proteins. The lumen's pH (~4.5–5.0) is optimal for the enzymes involved in hydrolysis, analogous to the activity of the stomach. Besides degradation of polymers, the lysosome is involved in various cell processes, including secretion, plasma membrane repair, apoptosis, cell signaling, and energy metabolism.

Lysosomal storage diseases are a group of over 70 rare inherited metabolic disorders that result from defects in lysosomal function. Lysosomes are sacs of enzymes within cells that digest large molecules and pass the fragments on to other parts of the cell for recycling. This process requires several critical enzymes. If one of these enzymes is defective due to a mutation, the large molecules accumulate within the cell, eventually killing it.

Autophagy is the natural, conserved degradation of the cell that removes unnecessary or dysfunctional components through a lysosome-dependent regulated mechanism. It allows the orderly degradation and recycling of cellular components. Although initially characterized as a primordial degradation pathway induced to protect against starvation, it has become increasingly clear that autophagy also plays a major role in the homeostasis of non-starved cells. Defects in autophagy have been linked to various human diseases, including neurodegeneration and cancer, and interest in modulating autophagy as a potential treatment for these diseases has grown rapidly.
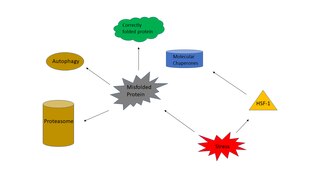
The heat shock response (HSR) is a cell stress response that increases the number of molecular chaperones to combat the negative effects on proteins caused by stressors such as increased temperatures, oxidative stress, and heavy metals. In a normal cell, proteostasis must be maintained because proteins are the main functional units of the cell. Many proteins take on a defined configuration in a process known as protein folding in order to perform their biological functions. If these structures are altered, critical processes could be affected, leading to cell damage or death. The heat shock response can be employed under stress to induce the expression of heat shock proteins (HSP), many of which are molecular chaperones, that help prevent or reverse protein misfolding and provide an environment for proper folding.

Heat shock 70 kDa protein 8 also known as heat shock cognate 71 kDa protein or Hsc70 or Hsp73 is a heat shock protein that in humans is encoded by the HSPA8 gene on chromosome 11. As a member of the heat shock protein 70 family and a chaperone protein, it facilitates the proper folding of newly translated and misfolded proteins, as well as stabilize or degrade mutant proteins. Its functions contribute to biological processes including signal transduction, apoptosis, autophagy, protein homeostasis, and cell growth and differentiation. It has been associated with an extensive number of cancers, neurodegenerative diseases, cell senescence, and aging.

The bafilomycins are a family of macrolide antibiotics produced from a variety of Streptomycetes. Their chemical structure is defined by a 16-membered lactone ring scaffold. Bafilomycins exhibit a wide range of biological activity, including anti-tumor, anti-parasitic, immunosuppressant and anti-fungal activity. The most used bafilomycin is bafilomycin A1, a potent inhibitor of cellular autophagy. Bafilomycins have also been found to act as ionophores, transporting potassium K+ across biological membranes and leading to mitochondrial damage and cell death.
In eukaryotic cells, an aggresome refers to an aggregation of misfolded proteins in the cell, formed when the protein degradation system of the cell is overwhelmed. Aggresome formation is a highly regulated process that possibly serves to organize misfolded proteins into a single location.
Lysosomal lipase is a form of lipase which functions intracellularly, in the lysosomes.

Lysosome-associated membrane protein 2 (LAMP2), also known as CD107b and Mac-3, is a human gene. Its protein, LAMP2, is one of the lysosome-associated membrane glycoproteins.

Vacuolar protein sorting ortholog 35 (VPS35) is a protein involved in autophagy and is implicated in neurodegenerative diseases, such as Parkinson's disease (PD) and Alzheimer's disease (AD). VPS35 is part of a complex called the retromer, which is responsible for transporting select cargo proteins between vesicular structures and the Golgi apparatus. Mutations in the VPS35 gene (VPS35) cause aberrant autophagy, where cargo proteins fail to be transported and dysfunctional or unnecessary proteins fail to be degraded. There are numerous pathways affected by altered VPS35 levels and activity, which have clinical significance in neurodegeneration. There is therapeutic relevance for VPS35, as interventions aimed at correcting VPS35 function are in speculation.

Transcription factor EB is a protein that in humans is encoded by the TFEB gene.

Lysosomal-associated transmembrane protein 4B is a protein that in humans is encoded by the LAPTM4B gene.
Proteostasis is the dynamic regulation of a balanced, functional proteome. The proteostasis network includes competing and integrated biological pathways within cells that control the biogenesis, folding, trafficking, and degradation of proteins present within and outside the cell. Loss of proteostasis is central to understanding the cause of diseases associated with excessive protein misfolding and degradation leading to loss-of-function phenotypes, as well as aggregation-associated degenerative disorders. Therapeutic restoration of proteostasis may treat or resolve these pathologies. Cellular proteostasis is key to ensuring successful development, healthy aging, resistance to environmental stresses, and to minimize homeostatic perturbations from pathogens such as viruses. Cellular mechanisms for maintaining proteostasis include regulated protein translation, chaperone assisted protein folding, and protein degradation pathways. Adjusting each of these mechanisms based on the need for specific proteins is essential to maintain all cellular functions relying on a correctly folded proteome.

David Sulzer is an American neuroscientist and musician. He is a professor at Columbia University Medical Center in the departments of psychiatry, neurology, and pharmacology. Sulzer's laboratory investigates the interaction between the synapses of the cerebral cortex and the basal ganglia, including the dopamine system, in habit formation, planning, decision making, and diseases of the system. His lab has developed the first means to optically measure neurotransmission, and has introduced new hypotheses of neurodegeneration in Parkinson's disease, and changes in synapses that produce autism and habit learning.

Chaperone-mediated autophagy (CMA) refers to the chaperone-dependent selection of soluble cytosolic proteins that are then targeted to lysosomes and directly translocated across the lysosome membrane for degradation. The unique features of this type of autophagy are the selectivity on the proteins that are degraded by this pathway and the direct shuttling of these proteins across the lysosomal membrane without the requirement for the formation of additional vesicles.
Chaperone-assisted selective autophagy is a cellular process for the selective, ubiquitin-dependent degradation of chaperone-bound proteins in lysosomes.
Microautophagy is one of the three common forms of autophagic pathway, but unlike macroautophagy and chaperone-mediated autophagy, it is mediated—in mammals by lysosomal action or in plants and fungi by vacuolar action—by direct engulfment of the cytoplasmic cargo. Cytoplasmic material is trapped in the lysosome/vacuole by a random process of membrane invagination.

QX39 is a synthetic compound that activates chaperone-mediated autophagy (CMA) by increasing the expression of the lysosomal receptor for this pathway, LAMP2A lysosomes. It showed potent activity in vitro but has poor pharmacokinetic properties and was not suitable for animal research. Subsequent research led to the development of CA77.1, a CMA activator suitable for in vivo use.
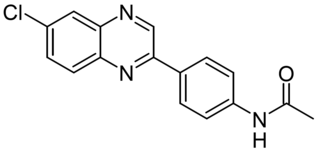
CA77.1 (CA) is a synthetic compound that activates chaperone-mediated autophagy (CMA) by increasing the expression of the lysosomal receptor for this pathway, LAMP2A, in lysosomes. CA77.1 is a derivative of earlier compound AR7(HY-101106), which shows potent CMA activation in vitro but is not suitable for in vivo use. CA77.1 is able to activate CMA in vivo, and demonstrates brain penetrance and favorable pharmacokinetics. It has been shown in animal studies that in vivo administration of CA77.1 to enhance chaperone-mediated autophagy, may help to degrade toxic pathogenic protein products such as tau proteins and has potential applications in the treatment of Alzheimer's disease particularly in improving both behavior and neuropathology in PS19 mice models.














