
Parkinsonism is a clinical syndrome characterized by tremor, bradykinesia, rigidity, and postural instability. Both hypokinetic as well as hyperkinetic features are displayed by Parkinsonism.These are the four motor symptoms found in Parkinson's disease (PD) – after which it is named – dementia with Lewy bodies (DLB), Parkinson's disease dementia (PDD), and many other conditions. This set of symptoms occurs in a wide range of conditions and may have many causes, including neurodegenerative conditions, drugs, toxins, metabolic diseases, and neurological conditions other than PD.

Dementia with Lewy bodies (DLB) is a type of dementia characterized by changes in sleep, behavior, cognition, movement, and regulation of automatic bodily functions. Memory loss is not always an early symptom. The disease worsens over time and is usually diagnosed when cognitive impairment interferes with normal daily functioning. Together with Parkinson's disease dementia, DLB is one of the two Lewy body dementias. It is a common form of dementia, but the prevalence is not known accurately and many diagnoses are missed. The disease was first described on autopsy by Kenji Kosaka in 1976, and he named the condition several years later.
Lewy body dementia is an umbrella term for two similar and common subtypes of dementia: dementia with Lewy bodies (DLB) and Parkinson's disease dementia (PDD). Both are characterized by changes in thinking, movement, behavior, and mood. The two conditions have similar features and may have similar causes, and are believed to belong on a spectrum of Lewy body disease that includes Parkinson's disease. As of 2014, they were more often misdiagnosed than any other common dementia.

Lewy bodies are the inclusion bodies – abnormal aggregations of protein – that develop inside nerve cells affected by Parkinson's disease (PD), the Lewy body dementias, and some other disorders. They are also seen in cases of multiple system atrophy, particularly the parkinsonian variant (MSA-P).

Alpha-synuclein (aSyn) is a protein that, in humans, is encoded by the SNCA gene. Alpha-synuclein is a neuronal protein that regulates synaptic vesicle trafficking and subsequent neurotransmitter release.

Multiple system atrophy (MSA) is a rare neurodegenerative disorder characterized by tremors, slow movement, muscle rigidity, and postural instability, autonomic dysfunction and ataxia. This is caused by progressive degeneration of neurons in several parts of the brain including the basal ganglia, inferior olivary nucleus, and cerebellum.

A neurodegenerative disease is caused by the progressive loss of structure or function of neurons, in the process known as neurodegeneration. Such neuronal damage may ultimately involve cell death. Neurodegenerative diseases include amyotrophic lateral sclerosis, multiple sclerosis, Parkinson's disease, Alzheimer's disease, Huntington's disease, multiple system atrophy, tauopathies, and prion diseases. Neurodegeneration can be found in the brain at many different levels of neuronal circuitry, ranging from molecular to systemic. Because there is no known way to reverse the progressive degeneration of neurons, these diseases are considered to be incurable; however research has shown that the two major contributing factors to neurodegeneration are oxidative stress and inflammation. Biomedical research has revealed many similarities between these diseases at the subcellular level, including atypical protein assemblies and induced cell death. These similarities suggest that therapeutic advances against one neurodegenerative disease might ameliorate other diseases as well.

Beta-synuclein is a protein that in humans is encoded by the SNCB gene.

Leucine-rich repeat kinase 2 (LRRK2), also known as dardarin and PARK8, is a large, multifunctional kinase enzyme that in humans is encoded by the LRRK2 gene. LRRK2 is a member of the leucine-rich repeat kinase family. Variants of this gene are associated with an increased risk of Parkinson's disease and Crohn's disease.

Probable cation-transporting ATPase 13A2 is an enzyme that in humans is encoded by the ATP13A2 gene that is involved in the transport of divalent transition metal cations. It appears to protect cells from manganese and zinc toxicity, possibly by causing cellular efflux and/or lysosomal sequestration; and from iron toxicity, possibly by preserving lysosome integrity against iron-induced lipid peroxidation. However, it potentiates the toxic effects of cadmium and nickel on developing neurites, and of the widely used herbicide paraquat possibly by increasing polyamine uptake.

Neurogenetics studies the role of genetics in the development and function of the nervous system. It considers neural characteristics as phenotypes, and is mainly based on the observation that the nervous systems of individuals, even of those belonging to the same species, may not be identical. As the name implies, it draws aspects from both the studies of neuroscience and genetics, focusing in particular how the genetic code an organism carries affects its expressed traits. Mutations in this genetic sequence can have a wide range of effects on the quality of life of the individual. Neurological diseases, behavior and personality are all studied in the context of neurogenetics. The field of neurogenetics emerged in the mid to late 20th century with advances closely following advancements made in available technology. Currently, neurogenetics is the center of much research utilizing cutting edge techniques.

Parkinson's disease (PD), or simply Parkinson's, is a long-term neurodegenerative disease of mainly the central nervous system that affects both the motor system and non-motor systems. The symptoms usually emerge slowly, and as the disease progresses, non-motor symptoms become more common. Usual symptoms are tremor, slowness of movement, rigidity, and difficulty with balance, collectively known as parkinsonism. Parkinson's disease dementia, falls and neuropsychiatric problems such as sleep abnormalities, psychosis, mood swings, or behavioral changes may arise in advanced stages.
Parkinson's disease (PD) is a complicated neurodegenerative disease that progresses over time and is marked by bradykinesia, tremor, and stiffness. As the condition worsens, some patients may also experience postural instability. Parkinson's disease (PD) is primarily caused by the gradual degeneration of dopaminergic neurons in the region known as the substantia nigra along with other monoaminergic cell groups throughout the brainstem, increased activation of microglia, and the build-up of Lewy bodies and Lewy neurites, which are proteins found in surviving dopaminergic neurons.

Alice M. Lazzarini is a scientist, author and researcher on neurogenetic disorders, including Huntington's disease and Parkinson's disease. She is an assistant professor of Neurology at Rutgers Robert Wood Johnson Medical School, where her work helped establish the genetic basis of Parkinson's. Later in life, she was diagnosed with Parkinson's—the very disease she had spent decades researching.
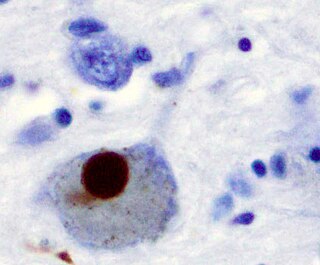
Synucleinopathies are neurodegenerative diseases characterised by the abnormal accumulation of aggregates of alpha-synuclein protein in neurons, nerve fibres or glial cells. There are three main types of synucleinopathy: Parkinson's disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA). Other rare disorders, such as various neuroaxonal dystrophies, also have α-synuclein pathologies. Additionally, autopsy studies have shown that around 6% of sporadic Alzheimer's Disease exhibit α-synuclein positive Lewy pathology, and are sub-classed as Alzheimer's Disease with Amygdalar Restricted Lewy Bodies (AD/ALB).
David Chaim Rubinsztein FRS FMedSci is the Deputy Director of the Cambridge Institute of Medical Research (CIMR), Professor of Molecular Neurogenetics at the University of Cambridge and a UK Dementia Research Institute Professor.

Rahul Desikan was an Indian-American neuroscientist and neuroradiologist. He was an Assistant Professor of Radiology & Biomedical Imaging, Neurology and Pediatrics at the University of California, San Francisco, and co-director of Laboratory for Precision Neuroimaging. Desikan's achievements became publicly known in a Washington Post article detailing his lifelong commitment to preventing and treating Alzheimer's disease and his continuing work as a scientist living with Amyotrophic lateral sclerosis (ALS). Desikan was vocal about the need for increased awareness and research funding for ALS, and voiced his unique perspective as both ALS researcher and ALS patient in op-ed articles appearing in a regular column in the Washington Post as well as in the San Francisco Chronicle and Scientific American.
Bryan J. Traynor is a neurologist and a senior investigator at the National Institute on Aging, and an adjunct professor at Johns Hopkins University. Dr. Traynor studies the genetics of human neurological conditions such as amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). He led the international consortium that identified pathogenic repeat expansions in the C9orf72 gene as a common cause of ALS and FTD. Dr. Traynor also led efforts that identified other Mendelian genes responsible for familial ALS and dementia, including VCP, MATR3, KIF5A, HTT, and SPTLC1.
Sonia Gandhi is a British physician and neuroscientist who leads the Francis Crick Institute neurodegeneration laboratory. She holds a joint position at the UCL Queen Square Institute of Neurology. Her research investigates the molecular mechanisms that give rise to Parkinson's disease. During the COVID-19 pandemic, Gandhi was involved with the epidemiological investigations and testing efforts at the Francis Crick Institute.
Christine Klein is a German physician who is a professor of neurology and neurogenetics at the University of Lübeck. Her research considers the molecular genetics of movement disorders. She is a Fellow of the European Academy of Neurology, former President of the German Neurological Society and incoming President of the European Section of the International Parkinson and Movement Disorder Society.














