Leishmania is a parasitic protozoan, a single-celled organism of the genus Leishmania that is responsible for the disease leishmaniasis. They are spread by sandflies of the genus Phlebotomus in the Old World, and of the genus Lutzomyia in the New World. At least 93 sandfly species are proven or probable vectors worldwide. Their primary hosts are vertebrates; Leishmania commonly infects hyraxes, canids, rodents, and humans.
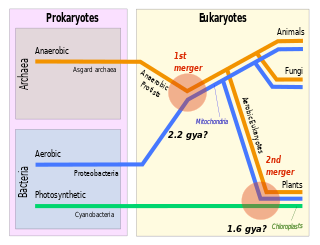
Symbiogenesis is the leading evolutionary theory of the origin of eukaryotic cells from prokaryotic organisms. The theory holds that mitochondria, plastids such as chloroplasts, and possibly other organelles of eukaryotic cells are descended from formerly free-living prokaryotes taken one inside the other in endosymbiosis. Mitochondria appear to be phylogenetically related to Rickettsiales bacteria, while chloroplasts are thought to be related to cyanobacteria.

Trypanosomatida is a group of kinetoplastid unicellular organisms distinguished by having only a single flagellum. The name is derived from the Greek trypano (borer) and soma (body) because of the corkscrew-like motion of some trypanosomatid species. All members are exclusively parasitic, found primarily in insects. A few genera have life-cycles involving a secondary host, which may be a vertebrate, invertebrate or plant. These include several species that cause major diseases in humans. Some trypanosomatida are intracellular parasites, with the important exception of Trypanosoma brucei.

Buchnera aphidicola, a member of the Pseudomonadota and the only species in the genus Buchnera, is the primary endosymbiont of aphids, and has been studied in the pea aphid, Acyrthosiphon pisum. Buchnera is believed to have had a free-living, Gram-negative ancestor similar to a modern Enterobacterales, such as Escherichia coli. Buchnera is 3 µm in diameter and has some of the key characteristics of its Enterobacterales relatives, such as a Gram-negative cell wall. However, unlike most other Gram-negative bacteria, Buchnera lacks the genes to produce lipopolysaccharides for its outer membrane. The long association with aphids and the limitation of crossover events due to strictly vertical transmission has seen the deletion of genes required for anaerobic respiration, the synthesis of amino sugars, fatty acids, phospholipids, and complex carbohydrates. This has resulted not only in one of the smallest known genomes of any living organism, but also one of the most genetically stable.
Mycoplasma hyopneumoniae is a species of bacteria known to cause the disease porcine enzootic pneumonia, a highly contagious and chronic disease affecting pigs. As with other mollicutes, M. hyopneumoniae is small in size (400–1200 nm), has a small genome and lacks a cell wall. It is difficult to grow in laboratories due to its complex nutritional requirements and the high chances of contamination associated with mycoplasma culture. To successfully grow the bacterium, an environment of 5–10% carbon dioxide is required, and the medium should demonstrate an acid colour shift.
Intracellular parasites are microparasites that are capable of growing and reproducing inside the cells of a host. They are also called intracellular pathogens.
Symbiotic bacteria are bacteria living in symbiosis with another organism or each other. For example, rhizobia living in root nodules of legumes provide nitrogen fixing activity for these plants.
Rhizopus microsporus is a fungal plant pathogen infecting maize, sunflower, and rice.
Crithidia fasciculata is a species of parasitic excavates. C. fasciculata, like other species of Crithidia have a single host life cycle with insect host, in the case of C. fasciculata this is the mosquito. C. fasciculata have low host species specificity and can infect many species of mosquito.
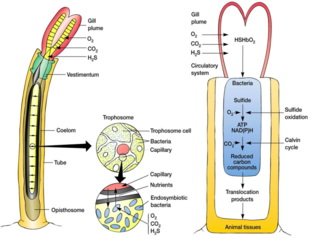
A trophosome is a highly vascularised organ found in some animals that houses symbiotic bacteria that provide food for their host. Trophosomes are contained by the coelom of the vestimentiferan tube worms and in the body of symbiotic flatworms of the genus Paracatenula.
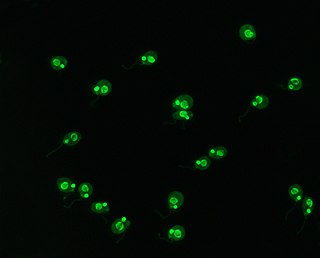
Crithidia luciliae is a flagellate parasite that uses the housefly, Musca domestica, as a host. As part of the family of Trypanosomatidae, it is characterised by the presence of a kinetoplast, a complex network of interlocking circular double-stranded DNA (dsDNA) molecules. The presence of the kinetoplast makes this organism important in the diagnosis of systemic lupus erythamatosus (SLE). By using C. luciliae as a substrate for immunofluorescence, the organelle can be used to detect anti-dsDNA antibodies, a common feature of the disease.
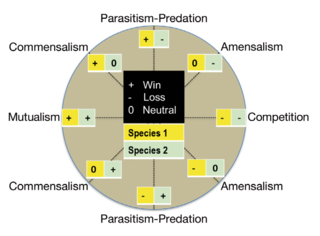
Microbial symbiosis in marine animals was not discovered until 1981. In the time following, symbiotic relationships between marine invertebrates and chemoautotrophic bacteria have been found in a variety of ecosystems, ranging from shallow coastal waters to deep-sea hydrothermal vents. Symbiosis is a way for marine organisms to find creative ways to survive in a very dynamic environment. They are different in relation to how dependent the organisms are on each other or how they are associated. It is also considered a selective force behind evolution in some scientific aspects. The symbiotic relationships of organisms has the ability to change behavior, morphology and metabolic pathways. With increased recognition and research, new terminology also arises, such as holobiont, which the relationship between a host and its symbionts as one grouping. Many scientists will look at the hologenome, which is the combined genetic information of the host and its symbionts. These terms are more commonly used to describe microbial symbionts.
The Scotokaryotes (Cavalier-Smith) is a proposed basal Neokaryote clade as sister of the Diaphoretickes. Basal Scotokaryote groupings are the Metamonads, the Malawimonas and the Podiata. In this phylogeny the Discoba are sometimes seen as paraphyletic and basal Eukaryotes.

A symbiosome is a specialised compartment in a host cell that houses an endosymbiont in a symbiotic relationship.

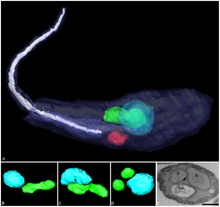
Strigomonas culicis is a protist and member of flagellated trypanosomatids. It is an obligate parasite in the gastrointestinal tract of mosquito, and is in turn a host to symbiotic bacteria. It maintains strict mutualistic relationship with the bacteria as a sort of cell organelle (endosymbiont) so that it cannot lead an independent life without the bacteria. Along with Angomonas deanei, S. culicis is researched as model organism for the evolution of symbiotic relationsships with intracellular bacteria.
Novymonas esmeraldas is a protist and member of flagellated trypanosomatids. It is an obligate parasite in the gastrointestinal tract of a bug, and is in turn a host to symbiotic bacteria. It maintains strict mutualistic relationship with the bacteria as a sort of cell organelle (endosymbiont) so that it cannot lead an independent life without the bacteria. Its discovery in 2016 suggests that it is a good model in the evolution of prokaryotes into eukaryotes by symbiogenesis. The endosymbiotic bacterium was identified as member of the genus Pandoraea.
Angomonas desouzai is a parasitic protist from the order Trypanosomatida.
Strigomonas oncopelti is a parasitic protist from the order Trypanosomatida.
Strigomonadinae is a subfamily of protists in the order Trypanosomatida. All species in this taxon harbor endodymbiontic bacteria of the Candidatus Kinetoplastibacterium genus.
Angomonas ambiguus is a parasitic protist from the order Trypanosomatida.