Related Research Articles

Natural killer cells, also known as NK cells or large granular lymphocytes (LGL), are a type of cytotoxic lymphocyte critical to the innate immune system. They belong to the rapidly expanding family of known innate lymphoid cells (ILC) and represent 5–20% of all circulating lymphocytes in humans. The role of NK cells is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to virus-infected cells, stressed cells, tumor cells, and other intracellular pathogens based on signals from several activating and inhibitory receptors. Most immune cells detect the antigen presented on major histocompatibility complex I (MHC-I) on infected cell surfaces, but NK cells can recognize and kill stressed cells in the absence of antibodies and MHC, allowing for a much faster immune reaction. They were named "natural killers" because of the notion that they do not require activation to kill cells that are missing "self" markers of MHC class I. This role is especially important because harmful cells that are missing MHC I markers cannot be detected and destroyed by other immune cells, such as T lymphocyte cells.
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies. Immunotherapy is under preliminary research for its potential to treat various forms of cancer.
A cancer vaccine, or oncovaccine, is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.
Lymphoid leukemias are a group of leukemias affecting circulating lymphocytes, a type of white blood cell. The lymphocytic leukemias are closely related to lymphomas of the lymphocytes, to the point that some of them are unitary disease entities that can be called by either name. Such diseases are all lymphoproliferative disorders. Most lymphoid leukemias involve a particular subtype of lymphocytes, the B cells.

Steven A. Rosenberg is an American cancer researcher and surgeon, chief of Surgery at the National Cancer Institute in Bethesda, Maryland and a Professor of Surgery at the Uniformed Services University of Health Sciences and the George Washington University School of Medicine and Health Sciences. He pioneered the development of immunotherapy that has resulted in the first effective immunotherapies and the development of gene therapy. He is the first researcher to successfully insert foreign genes into humans.

Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4, a protein receptor that downregulates the immune system.
In cell biology, a lymphokine-activated killer cell is a white blood cell that has been stimulated to kill tumor cells. If lymphocytes are cultured in the presence of Interleukin 2, it results in the development of effector cells which are cytotoxic to tumor cells.
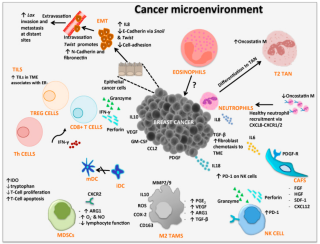
Cancer immunology (immuno-oncology) is an interdisciplinary branch of biology and a sub-discipline of immunology that is concerned with understanding the role of the immune system in the progression and development of cancer; the most well known application is cancer immunotherapy, which utilises the immune system as a treatment for cancer. Cancer immunosurveillance and immunoediting are based on protection against development of tumors in animal systems and (ii) identification of targets for immune recognition of human cancer.

Tumor-infiltrating lymphocytes (TIL) are white blood cells that have left the bloodstream and migrated towards a tumor. They include T cells and B cells and are part of the larger category of ‘tumor-infiltrating immune cells’ which consist of both mononuclear and polymorphonuclear immune cells, in variable proportions. Their abundance varies with tumor type and stage and in some cases relates to disease prognosis.
Immunotransplant is a maneuver used to make vaccines more powerful. It refers to the process of infusing vaccine-primed T lymphocytes into lymphodepleted recipients for the purpose of enhancing the proliferation and function of those T cells and increasing immune protection induced by that vaccine.
Cancer treatments are a wide range of treatments available for the many different types of cancer, with each cancer type needing its own specific treatment. Treatments can include surgery, chemotherapy, radiation therapy, hormonal therapy, targeted therapy including small-molecule drugs or monoclonal antibodies, and PARP inhibitors such as olaparib. Other therapies include hyperthermia, immunotherapy, photodynamic therapy, and stem-cell therapy. Most commonly cancer treatment involves a series of separate therapies such as chemotherapy before surgery. Angiogenesis inhibitors are sometimes used to enhance the effects of immunotherapies.
Adoptive cell transfer (ACT) is the transfer of cells into a patient. The cells may have originated from the patient or from another individual. The cells are most commonly derived from the immune system with the goal of improving immune functionality and characteristics. In autologous cancer immunotherapy, T cells are extracted from the patient, genetically modified and cultured in vitro and returned to the same patient. Comparatively, allogeneic therapies involve cells isolated and expanded from a donor separate from the patient receiving the cells.
Pelareorep is a proprietary isolate of the unmodified human reovirus being developed as a systemically administered immuno-oncological viral agent for the treatment of solid tumors and hematological malignancies. Pelareorep is an oncolytic virus, which means that it preferentially lyses cancer cells. Pelareorep also promotes an inflamed tumor phenotype through innate and adaptive immune responses. Preliminary clinical trials indicate that it may have anti-cancer effects across a variety of cancer types when administered alone and in combination with other cancer therapies.
ALECSAT technology is a novel method of epigenetic cancer immunotherapy being used by the company CytoVac. It uses a patient's own immune system to target tumor cells in prostate cancer, glioblastomas, and potentially pancreatic cancer. ALECSAT research, directed by Alexei Kirken and Karine Dzhandzhugazyan, has led to several clinical trials.
The NK-92 cell line is an immortalised cell line that has the characteristics of a type of immune cell found in human blood called ’natural killer’ (NK) cells. Blood NK cells and NK-92 cells recognize and attack cancer cells as well as cells that have been infected with a virus, bacteria, or fungus. NK-92 cells were first isolated in 1992 in the laboratory of Hans Klingemann at the British Columbia Cancer Agency in Vancouver, Canada, from a patient who had a rare NK cell non-Hodgkin-lymphoma. These cells were subsequently developed into a continuously growing cell line. NK-92 cells are distinguished by their suitability for expansion to large numbers, ability to consistently kill cancer cells and testing in clinical trials. When NK-92 cells recognize a cancerous or infected cell, they secrete perforin that opens holes into the diseased cells and releases granzymes that kill the target cells. NK-92 cells are also capable of producing cytokines such as tumor necrosis factor alpha (TNF-a) and interferon gamma (IFN-y), which stimulates proliferation and activation of other immune cells.
Cytokine-induced killer cells (CIK) cells are a group of immune effector cells featuring a mixed T- and natural killer (NK) cell-like phenotype. They are generated by ex vivo incubation of human peripheral blood mononuclear cells (PBMC) or cord blood mononuclear cells with interferon-gamma (IFN-γ), anti-CD3 antibody, recombinant human interleukin (IL)-1 and recombinant human interleukin (IL)-2.
Genelux Corporation is a late clinical-stage public company developing a pipeline of next-generation oncolytic viral immunotherapies for patients suffering from aggressive and/or difficult-to-treat solid tumor types. The Company’s most advanced product candidate, Olvi-Vec, is a proprietary, modified strain of the vaccinia virus (VACV), a stable DNA virus with a large engineering capacity.

Shimon Slavin is an Israeli professor of medicine. Slavin pioneered the use of immunotherapy mediated by allogeneic donor lymphocytes and innovative methods for stem cell transplantation for the cure of hematological malignancies and solid tumors, and using hematopoietic stem cells for induction of transplantation tolerance to bone marrow and donor allografts.

Cellular adoptive immunotherapy is a type of immunotherapy. Immune cells such as T-cells are usually isolated from patients for expansion or engineering purposes and reinfused back into patients to fight diseases using their own immune system. A major application of cellular adoptive therapy is cancer treatment, as the immune system plays a vital role in the development and growth of cancer. The primary types of cellular adoptive immunotherapies are T cell therapies. Other therapies include CAR-T therapy, CAR-NK therapy, macrophage-based immunotherapy and dendritic cell therapy.
References
- ↑ Rosenberg SA (January 1984). "Adoptive immunotherapy of cancer: accomplishments and prospects". Cancer Treat Rep. 68 (1): 233–55. PMID 6362866.
- ↑ Yang Q, Hokland ME, Bryant JL, et al. (July 2003). "Tumor-localization by adoptively transferred, interleukin-2-activated NK cells leads to destruction of well-established lung metastases". Int. J. Cancer. 105 (4): 512–9. doi:10.1002/ijc.11119. PMID 12712443. S2CID 25325594.
- 1 2 3 Egawa K (2004). "Immuno-cell therapy of cancer in Japan". Anticancer Res. 24 (5C): 3321–6. PMID 15515427.
- ↑ Kunishima S, Matsushita T, Shiratsuchi M, et al. (January 2005). "Detection of unique neutrophil non-muscle myosin heavy chain-A localization by immunofluorescence analysis in MYH9 disorder presented with macrothrombocytopenia without leukocyte inclusions and deafness". Eur. J. Haematol. 74 (1): 1–5. doi:10.1111/j.1600-0609.2004.00328.x. PMID 15613099. S2CID 20398097.
- 1 2 Manjunath, Sadananda Rao; Ramanan, Ganapathi; Dedeepiya, Vidyasagar Devaprasad; Terunuma, Hiroshi; Deng, Xuewen; Baskar, Subramani; Senthilkumar, Rajappa; Thamaraikannan, Paramasivam; Srinivasan, Thangavelu; Preethy, Senthilkumar; Abraham, Samuel J.K. (2012). "Autologous immune enhancement therapy in recurrent ovarian cancer with metastases; 18 months follow-up- A case report". Case Reports in Oncology. 5 (1): 114–118. doi:10.1159/000337319. PMC 3364094 . PMID 22666198.
- ↑ "Autologous immune enhancement therapy in recurrent ovarian cancer with metastases: a case report-Global Medical Discovery key scientific article". Archived from the original on 16 November 2012. Retrieved 30 August 2012.
- ↑ Fujita K, Ikarashi H, Takakuwa K, et al. (May 1995). "Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes". Clin. Cancer Res. 1 (5): 501–7. PMID 9816009.
- ↑ Kimura H, Yamaguchi Y (July 1997). "A phase III randomized study of interleukin-2 lymphokine-activated killer cell immunotherapy combined with chemotherapy or radiotherapy after curative or noncurative resection of primary lung carcinoma". Cancer. 80 (1): 42–9. doi:10.1002/(SICI)1097-0142(19970701)80:1<42::AID-CNCR6>3.0.CO;2-H. PMID 9210707.
- ↑ Takayama T, Sekine T, Makuuchi M, et al. (September 2000). "Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial". Lancet. 356 (9232): 802–7. doi:10.1016/S0140-6736(00)02654-4. PMID 11022927. S2CID 19241915.
- ↑ Kono K, Takahashi A, Ichihara F, et al. (June 2002). "Prognostic significance of adoptive immunotherapy with tumor-associated lymphocytes in patients with advanced gastric cancer: a randomized trial". Clin. Cancer Res. 8 (6): 1767–71. PMID 12060615.
- ↑ Sharat Damodar; Terunuma H; Sheriff AK; Farzana L; Manjunath S; Senthil KR; Shastikumar G; Abraham S (November 2006). "Autologous Immune Enhancement Therapy (AIET) for a Case of Acute Myeloid Leukemia (AML) – Our Experience". 1 (1): 40–41.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Baskar S, Dedeepiya V D, Terunuma H, Manjunath S R, Senthilkumar R, Sivaraman G, Pandian A, Abraham S. Prolonged survival of a patient with inoperable, locally advanced adenocarcinoma of pancreas after autologous immune enhancement therapy with chemotherapy. Indian J Cancer 2015;52:395-6.
- ↑ Sumana Premkumar; Vidyasagar Devaprasad Dedeepiya; Hiroshi Terunuma; Rajappa Senthilkumar; Thangavelu Srinivasan; Helen C. Reena; Senthilkumar Preethy; Samuel JK Abraham (2013). "Cell based Autologous Immune Enhancement Therapy (AIET) after radiotherapy in a locally advanced carcinoma of the cervix". Case Reports in Oncological Medicine. 2013: 1–3. doi: 10.1155/2013/903094 . PMC 3638504 . PMID 23653878.
- ↑ Press release on Advanced Ovarian Cancer treated with AIET
- ↑ Chidambaram R, Terunuma H, Balamurugan M, Dedeepiya VD, Sumana P, Senthilkumar R, Rajmohan M, Karthick R, Preethy S, Abraham SJK. Cell-based immunotherapy in stage IIIA inflammatory breast cancer with declining innate immunity following successive chemotherapies: A case report. Mol Clin Oncol. 2017 Sep;7(3):493–497.
- ↑ Revathi Raj; M. Deenadayalan; G. Vimal Kumar; Vipin Khandelwal; Karuna Sri; Rajappa Senthilkumar; Samuel J. K. Abraham; Terunuma Hiroshi (2014). "Autologous Immune Enhancement Therapy in Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia". Journal of Hematology and Blood Transfusion. 30 (Suppl 1): 202–204. doi:10.1007/s12288-013-0327-3. PMC 4192214 . PMID 25332578.
- ↑ Explore 'Killer Cell' Treatment to Cure rare Cancers
- ↑ Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K (November 2000). "Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population". Lancet. 356 (9244): 1795–9. doi:10.1016/S0140-6736(00)03231-1. PMID 11117911. S2CID 22918838.
- ↑ Vidyasagar Devaprasad Dedeepiya; Hiroshi Terunuma; Xuewen Deng; Subramani Baskar; Sadananda Rao Manjunath; Rajappa Senthilkumar; Palanisamy Murugan; Paramasivam Thamaraikannan; Thangavelu Srinivasan; Senthilkumar Preethy; Samuel J.K. Abraham (February 2012). "A comparative analysis of in vitro expansion of natural killer cells of a patient with autoimmune haemolytic anaemia and ovarian cancer with patients with other solid tumours". Oncology Letters. 3 (2): 435–440. doi:10.3892/ol.2011.498. PMC 3362377 . PMID 22740927.