Related Research Articles

Peroxyacetyl nitrate is a peroxyacyl nitrate. It is a secondary pollutant present in photochemical smog. It is thermally unstable and decomposes into peroxyethanoyl radicals and nitrogen dioxide gas. It is a lachrymatory substance, meaning that it irritates the lungs and eyes.

Ground-level ozone (O3), also known as surface-level ozone and tropospheric ozone, is a trace gas in the troposphere (the lowest level of the Earth's atmosphere), with an average concentration of 20–30 parts per billion by volume (ppbv), with close to 100 ppbv in polluted areas. Ozone is also an important constituent of the stratosphere, where the ozone layer (2 to 8 parts per million ozone) exists which is located between 10 and 50 kilometers above the Earth's surface. The troposphere extends from the ground up to a variable height of approximately 14 kilometers above sea level. Ozone is least concentrated in the ground layer (or planetary boundary layer) of the troposphere. Ground-level or tropospheric ozone is created by chemical reactions between NOx gases (oxides of nitrogen produced by combustion) and volatile organic compounds (VOCs). The combination of these chemicals in the presence of sunlight form ozone. Its concentration increases as height above sea level increases, with a maximum concentration at the tropopause. About 90% of total ozone in the atmosphere is in the stratosphere, and 10% is in the troposphere. Although tropospheric ozone is less concentrated than stratospheric ozone, it is of concern because of its health effects. Ozone in the troposphere is considered a greenhouse gas, and may contribute to global warming.

Nitrous acid is a weak and monoprotic acid known only in solution, in the gas phase and in the form of nitrite salts. Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagents in azo coupling reactions to give azo dyes.

Dinitrogen pentoxide is the chemical compound with the formula N2O5, also known as nitrogen pentoxide or nitric anhydride. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that melt at 41 °C. Its boiling point is 47 °C, and sublimes slightly above room temperature, yielding a colorless gas.

Atmospheric chemistry is a branch of atmospheric science in which the chemistry of the Earth's atmosphere and that of other planets is studied. It is a multidisciplinary approach of research and draws on environmental chemistry, physics, meteorology, computer modeling, oceanography, geology and volcanology and other disciplines. Research is increasingly connected with other areas of study such as climatology.
In atmospheric chemistry, NOx is shorthand for nitric oxide and nitrogen dioxide, the nitrogen oxides that are most relevant for air pollution. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone.
Soil chemistry is the study of the chemical characteristics of soil. Soil chemistry is affected by mineral composition, organic matter and environmental factors. In the early 1850s a consulting chemist to the Royal Agricultural Society in England, named J. Thomas Way, performed many experiments on how soils exchange ions, and is considered the father of soil chemistry. Other scientists who contributed to this branch of ecology include Edmund Ruffin, and Linus Pauling.

Over the last two centuries many environmental chemical observations have been made from a variety of ground-based, airborne, and orbital platforms and deposited in databases. Many of these databases are publicly available. All of the instruments mentioned in this article give online public access to their data. These observations are critical in developing our understanding of the Earth's atmosphere and issues such as climate change, ozone depletion and air quality. Some of the external links provide repositories of many of these datasets in one place. For example, the Cambridge Atmospheric Chemical Database, is a large database in a uniform ASCII format. Each observation is augmented with the meteorological conditions such as the temperature, potential temperature, geopotential height, and equivalent PV latitude.
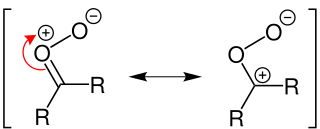
A Criegee intermediate is a carbonyl oxide with two charge centres. These chemicals may react with sulfur dioxide and nitrogen oxides in the earth's atmosphere, and are implicated in the formation of aerosols, which are an important factor in controlling global climate. Criegee intermediates are also an important source of OH. OH radicals are the most important oxidant in the troposphere, and are important in controlling air quality and pollution.

Iodine oxides are chemical compounds of oxygen and iodine. Iodine has only two stable oxides which are isolatable in bulk, iodine tetroxide and iodine pentoxide, but a number of other oxides are formed in trace quantities or have been hypothesized to exist. The chemistry of these compounds is complicated with only a few having been well characterized. Many have been detected in the atmosphere and are believed to be particularly important in the marine boundary layer.

SAGE III on ISS is the fourth generation of a series of NASA Earth-observing instruments, known as the Stratospheric Aerosol and Gas Experiment. The first SAGE III instrument was launched on a Russian Meteor-3M satellite. The recently revised SAGE III was mounted to the International Space Station where it uses the unique vantage point of ISS to make long-term measurements of ozone, aerosols, water vapor, and other gases in Earth's atmosphere.
In atmospheric chemistry, the Leighton relationship is an equation that determines the concentration of tropospheric ozone in areas polluted by the presence of nitrogen oxides. Ozone in the troposphere is primarily produced through the photolysis of nitrogen dioxide by photons with wavelengths (λ) less than 420 nanometers, which are able to reach the lowest levels of the atmosphere, through the following mechanism:
Heather Cecile Allen is a research chemist, who leads the Allen Group at Ohio State University. Allen's research focuses on interfacial phenomena, particularly those involving water and air. Her work has broad application ranging from medicine to climate change. She also develops nonlinear optical spectroscopy and microscopy instruments for the examination of interfacial surfaces.

Vicki Helene Grassian is the Chair of the Department of Chemistry and Biochemistry at the University of California, San Diego. She is also a Distinguished Professor in the Departments of Chemistry and Biochemistry, NanoEngineering, and Scripps Institution of Oceanography and holds the Distinguished Chair in Physical Chemistry.
Hind Al-Abadleh is a professor of chemistry at Wilfrid Laurier University in Waterloo, Ontario, Canada. She studies the physical chemistry of environmental interfaces, aerosols and climate change.
Jennifer G. Murphy is a Canadian environmental chemist and an associate professor at the University of Toronto. She is known for her research how air pollutants such as increased reactive nitrogen affect the global climate.

Nadine Unger is a Professor of Atmospheric Chemistry at the University of Exeter. She has studied the role of human activities and forests on the Earth's climate.

The bromine cycle is a biogeochemical cycle of bromine through the atmosphere, biosphere, and hydrosphere.
Jennifer Logan is an atmospheric scientist known for her research on how human activities influence the atmosphere, particularly with respect to biomass burning and the ozone hole.

CHIMERE is a chemistry-transport model. It is a computer code that unites a set of equations representing the transport and the chemistry of atmospheric species making it possible to quantify the evolution of air masses and pollution plumes as a function of time on different scales. Using meteorological inputs and emission fluxes, CHIMERE calculates three-dimensional concentrations of pollutants in the atmosphere. Due to the input data used, the number of equations that are solved and the physico-chemistry included in the model, CHIMERE is considered to be a mesoscale model, i.e. simulating the troposphere for a horizontal resolution of 1 to 100 km and over study areas ranging from the city to the hemisphere.
References
- 1 2 3 4 5 6 7 8 "Barbara J. Finlayson-Pitts". University of California Irvine - Faculty Profile System.
- ↑ Koppmann, Ralf (April 15, 2008). Volatile organic compounds in the atmosphere (1st ed.). Blackwell Pub. pp. 108–110, 147–148. ISBN 9780470988657 . Retrieved 16 October 2018.
- ↑ "Member Directory: Barbara Finlayson-Pitts". National Academy of Sciences. Retrieved 2022-03-22.
- ↑ "2017 National Award Recipients - American Chemical Society". American Chemical Society.
- ↑ Read "The Future of Atmospheric Chemistry Research: Remembering Yesterday, Understanding Today, Anticipating Tomorrow" at NAP.edu. 2016. doi:10.17226/23573. ISBN 978-0-309-44565-8.
- ↑ Finlayson-Pitts, B. J. (13 April 2010). "Atmospheric Chemistry". Proceedings of the National Academy of Sciences. 107 (15): 6566–6567. doi: 10.1073/pnas.1003038107 . PMC 2872471 . PMID 20388910.
- ↑ Richard, John P. (May 27, 2009). Advances in Physical Organic Chemistry. Vol. 43. Academic Press. pp. 85–87. ISBN 9780080886121 . Retrieved 16 October 2018.
- ↑ Kameda, Takayuki; Azumi, Eri; Fukushima, Aki; Tang, Ning; Matsuki, Atsushi; Kamiya, Yuta; Toriba, Akira; Hayakawa, Kazuichi (14 April 2016). "Mineral dust aerosols promote the formation of toxic nitropolycyclic aromatic compounds". Scientific Reports. 6 (1): 24427. Bibcode:2016NatSR...624427K. doi:10.1038/srep24427. PMC 4830986 . PMID 27075250.
- 1 2 3 4 Rosenzweig, Efrat (July 13, 2009). "Every Breath You Take". National Science Foundation. Retrieved 16 October 2018.
- ↑ Barboza, Tony (September 21, 2018). "87 days of smog: Southern California just saw its longest streak of bad air in decades". Los Angeles Times. Retrieved 16 October 2018.
- 1 2 "2007 Barbara Finlayson-Pitts, UC Irvine". Southern California Section of the American Chemical Society. 2012-07-17. Retrieved 16 October 2018.
- 1 2 Consensus Study Report (2016). "Chapter 3 Understanding Today". The future of atmospheric chemistry research : remembering yesterday, understanding today, anticipating tomorrow. National Academies Press. pp. 48–50. doi:10.17226/23573. ISBN 978-0309445658 . Retrieved 16 October 2018.
- 1 2 3 Raff, J. D.; Njegic, B.; Chang, W. L.; Gordon, M. S.; Dabdub, D.; Gerber, R. B.; Finlayson-Pitts, B. J. (20 July 2009). "Chlorine activation indoors and outdoors via surface-mediated reactions of nitrogen oxides with hydrogen chloride" (PDF). Proceedings of the National Academy of Sciences. 106 (33): 13647–13654. Bibcode:2009PNAS..10613647R. doi: 10.1073/pnas.0904195106 . PMC 2713392 . PMID 19620710.
- 1 2 Finlayson-Pitts, Barbara J. (February 2010). "Halogens in the Troposphere". Analytical Chemistry. 82 (3): 770–776. doi:10.1021/ac901478p. PMID 20041651.
- 1 2 Perraud, V.; Bruns, E. A.; Ezell, M. J.; Johnson, S. N.; Yu, Y.; Alexander, M. L.; Zelenyuk, A.; Imre, D.; Chang, W. L.; Dabdub, D.; Pankow, J. F.; Finlayson-Pitts, B. J. (30 January 2012). "Nonequilibrium atmospheric secondary organic aerosol formation and growth" (PDF). Proceedings of the National Academy of Sciences. 109 (8): 2836–2841. Bibcode:2012PNAS..109.2836P. doi: 10.1073/pnas.1119909109 . PMC 3286997 . PMID 22308444.
- ↑ Barringer, Felicity (February 18, 2012). "Scientists Find New Dangers in Tiny but Pervasive Particles in Air Pollution". The New York Times. Retrieved 16 October 2018.
- 1 2 Barboza, Tony (2014-06-25). "James Pitts dies at 93; his research led to cleaner air in California". Los Angeles Times . Retrieved 2014-07-18.
- ↑ "RSC Environment Prize 2019 Winner". www.rsc.org. Retrieved 2019-06-20.