Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides which have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.

Umami, or savoriness, is one of the five basic tastes. It has been described as savory and is characteristic of broths and cooked meats.

Glutamic acid is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABAergic neurons.

Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 that can be incorporated by special translation mechanisms.
The C-terminus is the end of an amino acid chain, terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus.
Neurophysin I is a carrier protein with a size of 10 KDa and contains 90 to 97 amino acids. It is a cleavage product of preprooxyphysin. It is a neurohypophysial hormone that is transported in vesicles with oxytocin, the other cleavage product, along axons, from magnocellular neurons of the hypothalamus to the posterior lobe of the pituitary. Although it is stored in neurosecretory granules with oxytocin and released with oxytocin, its biological action is unclear.
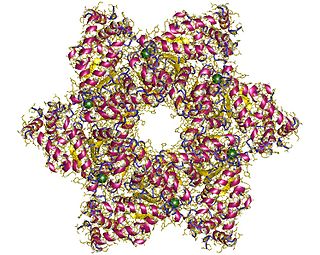
SV40 large T antigen is a hexamer protein that is a dominant-acting oncoprotein derived from the polyomavirus SV40. TAg is capable of inducing malignant transformation of a variety of cell types. The transforming activity of TAg is due in large part to its perturbation of the retinoblastoma (pRb) and p53 tumor suppressor proteins. In addition, TAg binds to several other cellular factors, including the transcriptional co-activators p300 and CBP, which may contribute to its transformation function. Similar proteins from related viruses are known as large tumor antigen in general.

Slotoxin is a peptide from Centruroides noxius Hoffmann scorpion venom. It belongs to the short scorpion toxin superfamily.

Neurophysin II is a carrier protein with a size of 19,687.3 Da and is made up of a dimer of two virtually identical chains of amino acids. Neurophysin II is a cleavage product of the AVP gene. It is a neurohypophysial hormone that is transported in vesicles with vasopressin, the other cleavage product, along axons, from magnocellular neurons of the hypothalamus to the posterior lobe of the pituitary. Although it is stored in neurosecretory granules with vasopressin and released with vasopressin into the bloodstream, its biological action is unclear. Neurophysin II is also known as a stimulator of prolactin secretion.
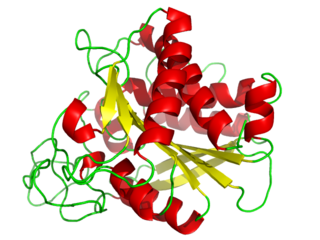
Carboxypeptidase A usually refers to the pancreatic exopeptidase that hydrolyzes peptide bonds of C-terminal residues with aromatic or aliphatic side-chains. Most scientists in the field now refer to this enzyme as CPA1, and to a related pancreatic carboxypeptidase as CPA2.
The discovery of an orally inactive peptide from snake venom established the unimportant role of angiotensin converting enzyme (ACE) inhibitors in regulating blood pressure. This led to the development of Captopril, the first ACE inhibitor. When the adverse effects of Captopril became apparent new derivates were designed. Then after the discovery of two active sites of ACE: N-domain and C-domain, the development of domain-specific ACE inhibitors began.
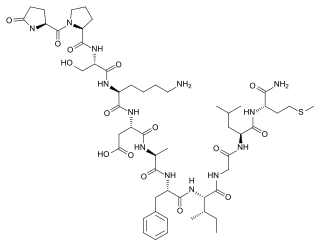
Eledoisin is an undecapeptide of mollusk origin, belonging to the tachykinin family of neuropeptides.

Xaa-Pro dipeptidase, also known as prolidase, is an enzyme that in humans is encoded by the PEPD gene.

Glutamate flavoring is the generic name for flavor-enhancing compounds based on glutamic acid and its salts (glutamates). These compounds provide an umami (savory) taste to food.
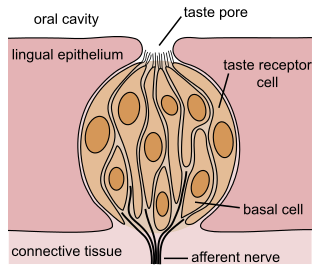
The gustatory system or sense of taste is the sensory system that is partially responsible for the perception of taste (flavor). Taste is the perception produced or stimulated when a substance in the mouth reacts chemically with taste receptor cells located on taste buds in the oral cavity, mostly on the tongue. Taste, along with olfaction and trigeminal nerve stimulation, determines flavors of food and other substances. Humans have taste receptors on taste buds and other areas, including the upper surface of the tongue and the epiglottis. The gustatory cortex is responsible for the perception of taste.
S-tag is the name of an oligopeptide derived from pancreatic ribonuclease A.
BeKm-1 is a toxin from the Central Asian scorpion Buthus eupeus. BeKm-1 acts by selectively inhibiting the human Ether-à-go-go Related Gene (hERG) channels, which are voltage gated potassium ion channels.
Leumorphin, also known as dynorphin B1–29, is a naturally occurring endogenous opioid peptide. Derived as a proteolytic cleavage product of residues 226-254 of prodynorphin, leumorphin is a nonacosapeptide and has the sequence Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Gln-Phe-Lys-Val-Val-Thr-Arg-Ser-Gln-Glu-Asp-Pro-Asn-Ala-Tyr-Ser-Gly-Glu-Leu-Phe-Asp-Ala. It can be further reduced to dynorphin B and dynorphin B-14 by pitrilysin metallopeptidase 1, an enzyme of the endopeptidase family. Leumorphin behaves as a potent and selective κ-opioid receptor agonist, similarly to other endogenous opioid peptide derivatives of prodynorphin.

Corn sauce or fermented corn sauce is produced by fermentation using corn starch as the primary substrate. It is used as a food condiment and ingredient, both in paste and in powder form. Corn sauce, like soy sauce, has a characteristic savory taste. It is used to flavor dishes including soups, broths, and gravies.
LmαTX5 is an α-scorpion toxin which inhibits the fast inactivation of voltage-gated sodium channels. It has been identified through transcriptome analysis of the venom gland of Lychas mucronatus, also known as the Chinese swimming scorpion – a scorpion species which is widely distributed in Southeast Asia.












