Related Research Articles
Polybrominated diphenyl ethers or PBDEs, are a class of organobromine compounds that are used as flame retardants. Like other brominated flame retardants, PBDEs have been used in a wide array of products, including building materials, electronics, furnishings, motor vehicles, airplanes, plastics, polyurethane foams, and textiles. They are structurally akin to polychlorinated diphenyl ethers (PCDEs), polychlorinated biphenyls (PCBs) and other polyhalogenated compounds, consisting of two halogenated aromatic rings. PBDEs are classified according to the average number of bromine atoms in the molecule. The health hazards of these chemicals have attracted increasing scrutiny, and they have been shown to reduce fertility in humans at levels found in households. Because of their toxicity and persistence, the industrial production of some PBDEs is restricted under the Stockholm Convention, a treaty to control and phase out major persistent organic pollutants (POPs).
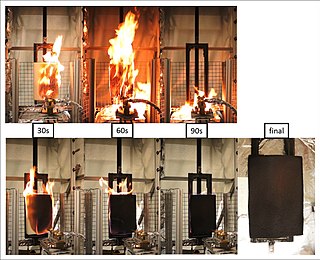
The term flame retardants subsumes a diverse group of chemicals that are added to manufactured materials, such as plastics and textiles, and surface finishes and coatings. Flame retardants are activated by the presence of an ignition source and are intended to prevent or slow the further development of ignition by a variety of different physical and chemical methods. They may be added as a copolymer during the polymerisation process, or later added to the polymer at a moulding or extrusion process or applied as a topical finish. Mineral flame retardants are typically additive while organohalogen and organophosphorus compounds can be either reactive or additive.
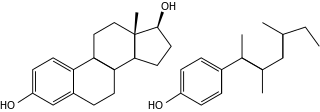
Endocrine disruptors, sometimes also referred to as hormonally active agents, endocrine disrupting chemicals, or endocrine disrupting compounds are chemicals that can interfere with endocrine systems. These disruptions can cause cancerous tumors, birth defects, and other developmental disorders. Found in many household and industrial products, endocrine disruptors "interfere with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body that are responsible for development, behavior, fertility, and maintenance of homeostasis ."
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is soluble in most common organic solvents, but has very poor solubility in water. BPA is produced on an industrial scale by the condensation of phenol and acetone, and has a global production scale which is expected to reach 10 million tonnes in 2022.

Polybrominated biphenyls (PBBs), also called brominated biphenyls or polybromobiphenyls, are a group of manufactured chemicals that consist of polyhalogenated derivatives of a biphenyl core. Their chlorine analogs are the PCBs. While once widely used commercially, PBBs are now controlled substances under the Restriction of Hazardous Substances Directive, which limits their use in electrical and electronic products sold in the EU.

A fire retardant is a substance that is used to slow down or stop the spread of fire or reduce its intensity. This is commonly accomplished by chemical reactions that reduce the flammability of fuels or delay their combustion. Fire retardants may also cool the fuel through physical action or endothermic chemical reactions. Fire retardants are available as powder, to be mixed with water, as fire-fighting foams and fire-retardant gels. Fire retardants are also available as coatings or sprays to be applied to an object.
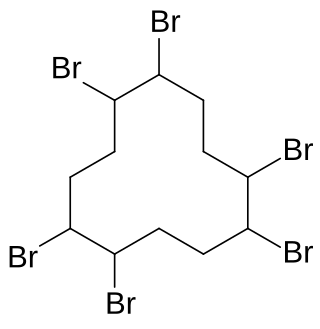
Hexabromocyclododecane is a brominated flame retardant. It consists of twelve carbon, eighteen hydrogen, and six bromine atoms tied to the ring. Its primary application is in extruded (XPS) and expanded (EPS) polystyrene foam that is used as thermal insulation in the building industry. Other uses are upholstered furniture, automobile interior textiles, car cushions and insulation blocks in trucks, packaging material, video cassette recorder housing and electric and electronic equipment. According to UNEP, "HBCD is produced in China, Europe, Japan, and the USA. The known current annual production is approximately 28,000 tonnes per year. The main share of the market volume is used in Europe and China". Due to its persistence, toxicity, and ecotoxicity, the Stockholm Convention on Persistent Organic Pollutants decided in May 2013 to list hexabromocyclododecane in Annex A to the convention with specific exemptions for production and use in expanded polystyrene and extruded polystyrene in buildings. Because HBCD has 16 possible stereo-isomers with different biological activities, the substance poses a difficult problem for manufacture and regulation.

Decabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). It was commercialised in the 1970s and was initially thought to be safe, but is now recognised as a hazardous and persistent pollutant. It was added to Annex A of the Stockholm Convention on Persistent Organic Pollutants in 2017, which means that treaty members must take measures to eliminate its production and use. The plastics industry started switching to decabromodiphenyl ethane as an alternative in the 1990s, but this is now also coming under regulatory pressure due to concerns over human health.
Pentabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). Because of their toxicity and persistence, their industrial production is to be eliminated under the Stockholm Convention, a treaty to control and phase out major persistent organic pollutants (POP).
Octabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs).
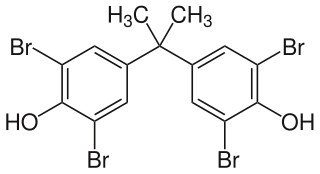
Tetrabromobisphenol A (TBBPA) is a brominated flame retardant. The compound is a white solid, although commercial samples appear yellow. It is one of the most common flame retardants.

Triphenyl phosphate (TPhP) is the chemical compound with the formula OP(OC6H5)3. This colourless solid is the ester (triester) of phosphoric acid and phenol. It is used as a plasticizer and a fire retardant in a wide variety of settings and products.
Ion-attachment mass spectrometry (IAMS) is a form of mass spectrometry that uses a "soft" form of ionization similar to chemical ionization in which a cation is attached to the analyte molecule in a reactive collision:

Diphenyl ether is the organic compound with the formula (C6H5)2O. It is a colorless solid. This, the simplest diaryl ether, has a variety of niche applications.
Organobromine compounds, also called organobromides, are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane.

In chemistry, congeners are chemical substances "related to each other by origin, structure, or function".

Susan D. Shaw was an American environmental health scientist, marine toxicologist, explorer, ocean conservationist, and author. A Doctor of Public Health, she was a professor in the Department of Environmental Health Sciences at the School of Public Health at the State University of New York at Albany, and Founder/President of the Shaw Institute, a nonprofit scientific institution with a mission to improve human and ecological health through innovative science and strategic partnerships. Shaw is globally recognized for pioneering high-impact environmental research on ocean pollution, climate change, oil spills, and plastics that has fueled public policy over three decades. In 1983, with landscape photographer Ansel Adams, she published Overexposure, the first book to document the health hazards of photographic chemicals. Shaw is credited as the first scientist to show that brominated flame retardant chemicals used in consumer products have contaminated marine mammals and commercially important fish stocks in the northwest Atlantic Ocean. She became the first scientist to dive into the Gulf of Mexico oil slick following the 2010 BP Deepwater Horizon oil rig explosion to investigate the impacts of chemical dispersants used in response to the spill.
The Shaw Institute, formerly the Marine & Environmental Research Institute, is a 501(c)(3) nonprofit scientific research organization based in Blue Hill, Maine and New York City. The Institute conducts research into ocean pollution, flame retardants, microplastics and plastic pollution, sentinel species and climate change.
Diana S. Aga is a Filipino-American chemist who is the Henry M. Woodburn Chair at the University at Buffalo. She was awarded the 2017 American Chemical Society Schoellkopf Medal in recognition of her work in environmental chemistry. The Schoellkopf Medal is a local section award of the American Chemical Society. Over the years, numerous University at Buffalo faculty have received this recognition.

Decabromodiphenyl ethane is a chemical compound used as a brominated flame retardant. It was commercialised in the 1990s as an alternative for decabromodiphenyl ether, following safety concern over that compound. The two molecules are chemically very similar, which gives them a similar application profile. Decabromodiphenyl ethane is now also coming under regulatory pressure.
References
- ↑ Michael J. Dagani, Henry J. Barda, Theodore J. Benya, David C. Sanders: Bromine Compounds, Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi : 10.1002/14356007.a04_405
- ↑ The European Commission (9 February 2017). "Commission Regulation (EU) 2017/227". Official Journal of the European Union. L35: 6–9. Retrieved 16 June 2017.
- ↑ The final decision is available on the UNEP Stockholm Convention website here: "COP Decisions". Archived from the original on 2014-09-25. Retrieved 2014-10-26.
- ↑ EU Risk Assessment Report (2006) "Archived copy" (PDF). Archived from the original (PDF) on 2014-09-05. Retrieved 2014-10-26.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Pedro Arias (2001): Brominated flame retardants – an overview. The Second International Workshop on Brominated Flame Retardants, Stockholm
- ↑ Townsend Solutions Estimate, "Flammschutz Online - the flame retardants market". Archived from the original on 2016-03-04. Retrieved 2014-10-26.
- ↑ Stiffler, Lisa (March 28, 2007). "PBDEs: They are everywhere, they accumulate and they spread". Seattle Post Intelligencer .
- ↑ Kim Hooper; Jianwen She (2003). "Lessons from the Polybrominated Diphenyl Ethers (PBDEs): Precautionary Principle, Primary Prevention, and the Value of Community-Based Body-Burden Monitoring Using Breast Milk". Environmental Health Perspectives . 111 (1): 109–114. doi:10.1289/ehp.5438. PMC 1241314 . PMID 12515688. Archived from the original on 2008-11-01.
- ↑ "Polybrominated Diphenyl Ethers (PBDEs) Action Plan Summary | Existing Chemicals | OPPT | US EPA". Archived from the original on 2015-09-01. Retrieved 2012-12-03.
- ↑ "Archived copy" (PDF). Archived (PDF) from the original on 2016-05-08. Retrieved 2012-12-03.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Shaw, Susan D. (2013). "Persistent organic pollutants including polychlorinated and polybrominated dibenzo-p-dioxins and dibenzofurans in firefighters from Northern California". Chemosphere. 91 (10): 1386–1394. Bibcode:2013Chmsp..91.1386S. doi:10.1016/j.chemosphere.2012.12.070. PMID 23395527.
- ↑ European Union Risk Assessment Report of diphenyl ether, pentabromo deriv., 2000 "Archived copy". Archived from the original on 2014-10-26. Retrieved 2014-10-26.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ European Union Risk Assessment Report of diphenyl ether, octabromo deriv., 2003 "Archived copy". Archived from the original on 2014-10-26. Retrieved 2014-10-26.
{{cite web}}: CS1 maint: archived copy as title (link)