
Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from the Ancient Greek βρῶμος (bromos) meaning "stench", referring to its sharp and pungent smell.

Toluene, also known as toluol, is a substituted aromatic hydrocarbon with the chemical formula C6H5CH3, often abbreviated as PhCH3, where Ph stands for phenyl group. It is a colorless, water-insoluble liquid with the odor associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group by a single bond. As such, its systematic IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent.
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring.

Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) with formula C14H10, consisting of three fused benzene rings. It is a colorless, crystal-like solid, but can also appear yellow. Phenanthrene is used to make dyes, plastics, pesticides, explosives, and drugs. It has also been used to make bile acids, cholesterol and steroids.
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.
In organic chemistry, an aryl halide is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications.

Hydrogen bromide is the inorganic compound with the formula HBr. It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temperature. Aqueous solutions that are 47.6% HBr by mass form a constant-boiling azeotrope mixture that boils at 124.3 °C (255.7 °F). Boiling less concentrated solutions releases H2O until the constant-boiling mixture composition is reached.
In chemistry, a trimer is a molecule or polyatomic anion formed by combination or association of three molecules or ions of the same substance. In technical jargon, a trimer is a kind of oligomer derived from three identical precursors often in competition with polymerization.
In organic chemistry, an electrophilic aromatic halogenation is a type of electrophilic aromatic substitution. This organic reaction is typical of aromatic compounds and a very useful method for adding substituents to an aromatic system.
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. It is an example of a radical-nucleophilic aromatic substitution. The Sandmeyer reaction provides a method through which one can perform unique transformations on benzene, such as halogenation, cyanation, trifluoromethylation, and hydroxylation.
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.

A boronic acid is an organic compound related to boric acid in which one of the three hydroxyl groups is replaced by an alkyl or aryl group. As a compound containing a carbon–boron bond, members of this class thus belong to the larger class of organoboranes.
In organic synthesis, cyanation is the attachment or substitution of a cyanide group on various substrates. Such transformations are high-value because they generate C-C bonds. Furthermore nitriles are versatile functional groups.
Organobromine chemistry is the study of the synthesis and properties of organobromine compounds, also called organobromides, which are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane.
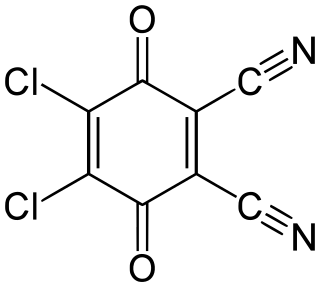
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (or DDQ) is the chemical reagent with formula C6Cl2(CN)2O2. This oxidant is useful for the dehydrogenation of alcohols, phenols, and steroid ketones. DDQ decomposes in water, but is stable in aqueous mineral acid.

Propargyl bromide, also known as 3-bromo-prop-1-yne, is an organic compound with the chemical formula HC≡CCH2Br. A colorless liquid, it is a halogenated organic compound consisting of propyne with a bromine substituent on the methyl group. It has a lachrymatory effect, like related compounds. The compound is used as a reagent in organic synthesis.
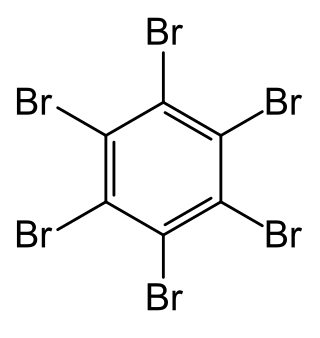
Hexabromobenzene is an aryl bromide and a six-substituted bromobenzene in which all six positions of the central benzene ring are bonded to a bromine atom.

4-Fluorobromobenzene is a mixed aryl halide (aryl fluoride and aryl bromide) with the formula C6H4BrF. It is a derivative of benzene, with a bromine atom bonded para to a fluorine atom. It has uses as a precursor to some pharmaceuticals, as an agrochemical intermediate, and in organic synthesis. It is a colorless liquid of low acute toxicity.

Pentaphenylantimony is an organoantimony compound containing five phenyl groups attached to one antimony atom. It has formula Sb(C6H5)5 (or SbPh5).















