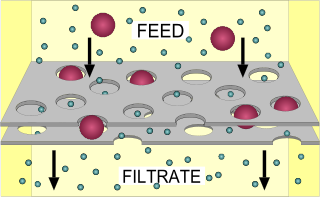
Filtration is a physical, biological or chemical operation that separates solid matter and fluid from a mixture with a filter medium that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate. Oversize particles may form a filter cake on top of the filter and may also block the filter lattice, preventing the fluid phase from crossing the filter, known as blinding. The size of the largest particles that can successfully pass through a filter is called the effective pore size of that filter. The separation of solid and fluid is imperfect; solids will be contaminated with some fluid and filtrate will contain fine particles. Filtration occurs both in nature and in engineered systems; there are biological, geological, and industrial forms.

Food additives are substances added to food to preserve flavor or enhance its taste, appearance, or other qualities. Some additives have been used for centuries; for example, preserving food by pickling, salting, as with bacon, preserving sweets or using sulfur dioxide as with wines. With the advent of processed foods in the second half of the twentieth century, many more additives have been introduced, of both natural and artificial origin. Food additives also include substances that may be introduced to food indirectly in the manufacturing process, through packaging, or during storage or transport.

A detergent is a surfactant or a mixture of surfactants with cleansing properties in dilute solutions. These substances are usually alkylbenzenesulfonates, a family of compounds that are similar to soap but are more soluble in hard water, because the polar sulfonate is less likely than the polar carboxylate to bind to calcium and other ions found in hard water.
A leaven, often called a leavening agent, is any one of a number of substances used in doughs and batters that cause a foaming action that lightens and softens the mixture. An alternative or supplement to leavening agents is mechanical action by which air is incorporated. Leavening agents can be biological or synthetic chemical compounds. The gas produced is often carbon dioxide, or occasionally hydrogen.

Powdered sugar, also called confectioners' sugar or icing sugar, is a finely ground sugar produced by milling granulated sugar into a powdered state. It usually contains a small amount of anti-caking agent to prevent clumping and improve flow. Although most often produced in a factory, powdered sugar can also be made by processing ordinary granulated sugar in a coffee grinder, or by crushing it by hand in a mortar and pestle.

Silica gel is an amorphous and porous form of silicon dioxide (silica), consisting of an irregular tridimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores. The voids may contain water or some other liquids, or may be filled by gas or vacuum. In the latter case, the material is properly called Silica xerogel.

Sodium triphosphate (STP), also sodium tripolyphosphate (STPP), or tripolyphosphate (TPP),) is an inorganic compound with formula Na5P3O10. It is the sodium salt of the polyphosphate penta-anion, which is the conjugate base of triphosphoric acid. It is produced on a large scale as a component of many domestic and industrial products, especially detergents. Environmental problems associated with eutrophication are attributed to its widespread use.
Flocculation, in the field of chemistry, is a process in which colloids come out of suspension in the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from precipitation in that, prior to flocculation, colloids are merely suspended in a liquid and not actually dissolved in a solution. In the flocculated system, there is no formation of a cake, since all the flocs are in the suspension.
Chemical changes occur when a substance combines with another to form a new substance, called chemical synthesis or, alternatively, chemical decomposition into two or more different substances. These processes are called chemical reactions and, in general, are not reversible except by further chemical reactions. Some reactions produce heat and are called exothermic reactions and others may require heat to enable the reaction to occur, which are called endothermic reactions. Understanding chemical changes is a major part of the science of chemistry.
An anticaking agent is an additive placed in powdered or granulated materials, such as table salt or confectioneries, to prevent the formation of lumps (caking) and for easing packaging, transport, flowability, and consumption. Caking mechanisms depend on the nature of the material. Crystalline solids often cake by formation of liquid bridge and subsequent fusion of microcrystals. Amorphous materials can cake by glass transitions and changes in viscosity. Polymorphic phase transitions can also induce caking.
A binder or binding agent is any material or substance that holds or draws other materials together to form a cohesive whole mechanically, chemically, by adhesion or cohesion.
Sodium aluminosilicate refers to compounds which contain sodium, aluminium, silicon and oxygen, and which may also contain water. These include synthetic amorphous sodium aluminosilicate, a few naturally occurring minerals and synthetic zeolites. Synthetic amorphous sodium aluminosilicate is widely used as a food additive, E 554.

Laundry detergent, or washing powder, is a type of detergent used for cleaning laundry. Laundry detergent is manufactured in powder and liquid form.

Bath salts are water-soluble, pulverized minerals that are added to water to be used for bathing. They are said to improve cleaning, enhance the enjoyment of bathing, and serve as a vehicle for cosmetic agents. Bath salts have been developed which mimic the properties of natural mineral baths or hot springs. Some bath salts contain glycerine so the product will act as an emollient, humectant or lubricant. Fragrances and colors are often added to bath salts; the fragrances are used to increase users' enjoyment of the bathing experience.

Disodium phosphate (DSP), or sodium hydrogen phosphate, or sodium phosphate dibasic, is the inorganic compound with the formula Na2HPO4. It is one of several sodium phosphates. The salt is known in anhydrous form as well as forms with 2, 7, 8, and 12 hydrates. All are water-soluble white powders; the anhydrous salt being hygroscopic.
A dispersant or a dispersing agent or a plasticizer or a superplasticizer is either a non-surface active polymer or a surface-active substance added to a suspension, usually a colloid, to improve the separation of particles and to prevent settling or clumping. Dispersants consist normally of one or more surfactants.
Cleaning agents or hard-surface cleaners are substances used to remove dirt, including dust, stains, bad smells, and clutter on surfaces. Purposes of cleaning agents include health, beauty, removing offensive odor, and avoiding the spread of dirt and contaminants to oneself and others. Some cleaning agents can kill bacteria and clean at the same time. Others, called degreasers, contain organic solvents to help dissolve oils and fats.
Granulation is the process of forming grains or granules from a powdery or solid substance, producing a granular material. It is applied in several technological processes in the chemical and pharmaceutical industries. Typically, granulation involves agglomeration of fine particles into larger granules, typically of size range between 0.2 and 4.0 mm depending on their subsequent use. Less commonly, it involves shredding or grinding solid material into finer granules or pellets.
Synthetic magnesium silicates are white, odorless, finely divided powders formed by the precipitation reaction of water-soluble sodium silicate and a water-soluble magnesium salt such as magnesium chloride, magnesium nitrate or magnesium sulfate. The composition of the precipitate depends on the ratio of the components in the reaction medium, the addition of the correcting substances, and the way in which they are precipitated.

Dishwasher detergent is a detergent made for washing dishes in a dishwasher. Dishwasher detergent is different from dishwashing liquid made to wash dishes by hand.











