Related Research Articles
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells. It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into many compartments.
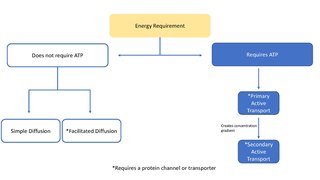
In cellular biology, active transport is the movement of molecules across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient. Active transport requires cellular energy to achieve this movement. There are two types of active transport: primary active transport that uses adenosine triphosphate (ATP), and secondary active transport that uses an electrochemical gradient. Active transport does not follow Fick's first law and Second law of thermodynamics

ATPases (EC 3.6.1.3, adenylpyrophosphatase, ATP monophosphatase, triphosphatase, SV40 T-antigen, adenosine 5'-triphosphatase, ATP hydrolase, complex V (mitochondrial electron transport), (Ca2+ + Mg2+)-ATPase, HCO3−-ATPase, adenosine triphosphatase) are a class of enzymes that catalyze the decomposition of ATP into ADP and a free phosphate ion or the inverse reaction. This dephosphorylation reaction releases energy, which the enzyme (in most cases) harnesses to drive other chemical reactions that would not otherwise occur. This process is widely used in all known forms of life.
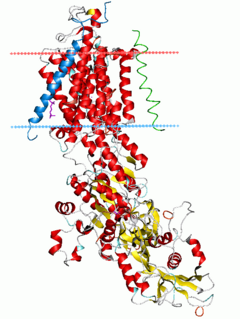
Na⁺/K⁺-ATPase is an enzyme found in the membrane of all animal cells. It performs several functions in cell physiology.
SERCA, or sarco/endoplasmic reticulum Ca2+-ATPase, or SR Ca2+-ATPase, is a calcium ATPase-type P-ATPase. Its major function is to transport calcium from the cytosol into the sarcoplasmic reticulum.

Astrocytes, also known collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord. They perform many functions, including biochemical support of endothelial cells that form the blood–brain barrier, provision of nutrients to the nervous tissue, maintenance of extracellular ion balance, regulation of cerebral blood flow, and a role in the repair and scarring process of the brain and spinal cord following infection and traumatic injuries. The proportion of astrocytes in the brain is not well defined; depending on the counting technique used, studies have found that the astrocyte proportion varies by region and ranges from 20% to 40% of all glia. Another study reports that astrocytes are the most numerous cell type in the brain. Astrocytes are the major source of cholesterol in the central nervous system. Apolipoprotein E transports cholesterol from astrocytes to neurons and other glial cells, regulating cell signaling in the brain. Astrocytes in humans are more than twenty times larger than in rodent brains, and make contact with more than ten times the number of synapses.

In excitotoxicity, nerve cells suffer damage or death when the levels of otherwise necessary and safe neurotransmitters such as glutamate, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), or N-methyl-D-aspartic acid (NMDA) become pathologically high resulting in excessive stimulation of receptors. For example, when glutamate receptors such as the NMDA receptor or AMPA receptor encounter excessive levels of the excitatory neurotransmitter glutamate significant neuronal damage might ensue. Excess glutamate allows high levels of calcium ions (Ca2+) to enter the cell. Ca2+ influx into cells activates a number of enzymes, including phospholipases, endonucleases, and proteases such as calpain. These enzymes go on to damage cell structures such as components of the cytoskeleton, membrane, and DNA. In evolved, complex adaptive systems such as biologic life it must be understood that mechanisms are rarely, if ever, simplistically direct. For example, NMDA in subtoxic amounts induces neuronal survival to otherwise toxic levels of glutamate.
A nanodomain is a nanometer-sized cluster of proteins found in a cell membrane. They are associated with the signal which occurs when a single calcium ion channel opens on a cell membrane, allowing an influx of calcium ions (Ca2+) which extend in a plume a few tens of nanometres from the channel pore. In a nanodomain, the coupling distance, that is, the distance between the calcium-binding proteins which sense the calcium, and the calcium channel, is very small, less than 100 nm (3.9×10−6 in), which allows rapid signalling. The formation of a nanodomain signal is virtually instantaneous following the opening of the calcium channel, as calcium ions move rapidly into the cell along a steep concentration gradient. The nanodomain signal collapses just as quickly when the calcium channel closes, as the ions rapidly diffuse away from the pore. Formation of a nanodomain signal requires the influx of only approximately 1000 calcium ions.
Glutamate transporters are a family of neurotransmitter transporter proteins that move glutamate – the principal excitatory neurotransmitter – across a membrane. The family of glutamate transporters is composed of two primary subclasses: the excitatory amino acid transporter (EAAT) family and vesicular glutamate transporter (VGLUT) family. In the brain, EAATs remove glutamate from the synaptic cleft and extrasynaptic sites via glutamate reuptake into glial cells and neurons, while VGLUTs move glutamate from the cell cytoplasm into synaptic vesicles. Glutamate transporters also transport aspartate and are present in virtually all peripheral tissues, including the heart, liver, testes, and bone. They exhibit stereoselectivity for L-glutamate but transport both L-aspartate and D-aspartate.
In biology, a transporter is a transmembrane protein that moves ions across a biological membrane to accomplish many different biological functions including, cellular communication, maintaining homeostasis, energy production, etc. There are different types of transporters including, pumps, uniporters, antiporters, and symporters. Active transporters or ion pumps are transporters that convert energy from various sources—including adenosine triphosphate (ATP), sunlight, and other redox reactions—to potential energy by pumping an ion up its concentration gradient. This potential energy could then be used by secondary transporters, including ion carriers and ion channels, to drive vital cellular processes, such as ATP synthesis.
Gastric hydrogen potassium ATPase, also known as H+/K+ ATPase, is an enzyme which functions to acidify the stomach. It is a member of the P-type ATPases, also known as E1-E2 ATPases due to its two states.
The sodium-calcium exchanger (often denoted Na+/Ca2+ exchanger, exchange protein, or NCX) is an antiporter membrane protein that removes calcium from cells. It uses the energy that is stored in the electrochemical gradient of sodium (Na+) by allowing Na+ to flow down its gradient across the plasma membrane in exchange for the countertransport of calcium ions (Ca2+). A single calcium ion is exported for the import of three sodium ions. The exchanger exists in many different cell types and animal species. The NCX is considered one of the most important cellular mechanisms for removing Ca2+.

Ca2+ ATPase is a form of P-ATPase that transfers calcium after a muscle has contracted. The two kinds of calcium ATPase are:

The plasma membrane Ca2+ ATPase (PMCA) is a transport protein in the plasma membrane of cells and functions to remove calcium (Ca2+) from the cell. PMCA function is vital for regulating the amount of Ca2+ within all eukaryotic cells. There is a very large transmembrane electrochemical gradient of Ca2+ driving the entry of the ion into cells, yet it is very important that they maintain low concentrations of Ca2+ for proper cell signalling. Thus, it is necessary for cells to employ ion pumps to remove the Ca2+. The PMCA and the sodium calcium exchanger (NCX) are together the main regulators of intracellular Ca2+ concentrations. Since it transports Ca2+ into the extracellular space, the PMCA is also an important regulator of the calcium concentration in the extracellular space.
Membrane channels are a family of biological membrane proteins which allow the passive movement of ions, water (aquaporins) or other solutes to passively pass through the membrane down their electrochemical gradient. They are studied using a range of channelomics experimental and mathematical techniques. Insights have suggested endocannabinoids (eCBs) as molecules that can regulate the opening of these channels during diverse conditions.
Gliotransmitters are chemicals released from glial cells that facilitate neuronal communication between neurons and other glial cells. They are usually induced from Ca2+ signaling, although recent research has questioned the role of Ca2+ in gliotransmitters and may require a revision of the relevance of gliotransmitters in neuronal signalling in general.
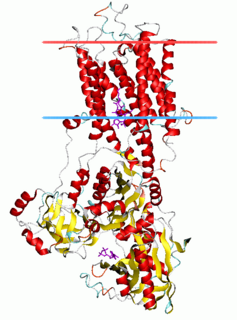
The P-type ATPases, also known as E1-E2 ATPases, are a large group of evolutionarily related ion and lipid pumps that are found in bacteria, archaea, and eukaryotes. P-type ATPases are α-helical bundle primary transporters named based upon their ability to catalyze auto- (or self-) phosphorylation (hence P) of a key conserved aspartate residue within the pump and their energy source, adenosine triphosphate (ATP). In addition, they all appear to interconvert between at least two different conformations, denoted by E1 and E2. P-type ATPases fall under the P-type ATPase (P-ATPase) Superfamily (TC# 3.A.3) which, as of early 2016, includes 20 different protein families.
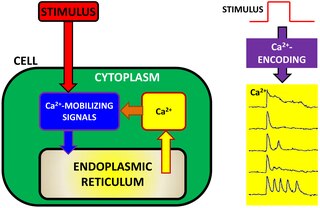
Calcium encoding (also referred to as Ca2+ encoding or calcium information processing) is an intracellular signaling pathway used by many cells to transfer, process and encode external information detected by the cell. In cell physiology, external information is often converted into intracellular calcium dynamics. The concept of calcium encoding explains how Ca2+ ions act as intracellular messengers, relaying information within cells to regulate their activity. Given the ubiquity of Ca2+ ions in cell physiology, Ca2+ encoding has also been suggested as a potential tool to characterize cell physiology in health and disease. The mathematical bases of Ca2+ encoding have been pioneered by work of Joel Keizer and Hans G. Othmer on calcium modeling in the 1990s and more recently they have been revisited by Eshel Ben-Jacob, Herbert Levine and co-workers.
Calcium imaging is a microscopy technique to optically measure the calcium (Ca2+) status of an isolated cell, tissue or medium. Calcium imaging takes advantage of calcium indicators, fluorescent molecules that respond to the binding of Ca2+ ions by fluorescence properties. Two main classes of calcium indicators exist: chemical indicators and genetically encoded calcium indicators (GECI). This technique has allowed studies of calcium signalling in a wide variety of cell types. In neurons, electrical activity is always accompanied by an influx of Ca2+ ions. Thus, calcium imaging can be used to monitor the electrical activity in hundreds of neurons in cell culture or in living animals, which has made it possible to dissect the function of neuronal circuits.

Alexei Verkhratsky, sometimes spelled Alexej, is a professor of neurophysiology at the University of Manchester best known for his research on the physiology and pathophysiology of neuroglia, calcium signalling, and brain ageing. He is an elected member and vice-president of Academia Europaea, of the German National Academy of Sciences Leopoldina, of the Real Academia Nacional de Farmacia (Spain), of the Slovenian Academy of Sciences and Arts, of Polish Academy of Sciences, and Dana Alliance for Brain Initiatives, among others. Since 2010. he is a Ikerbasque Research Professor and from 2012 he is deputy director of the Achucarro Basque Center for Neuroscience in Bilbao. He is a distinguished professor at Jinan University, China Medical University of Shenynag, and Chengdu University of Traditional Chinese Medicine and is an editor-in-chief of Cell Calcium, receiving editor for Cell Death and Disease, and Acta Physiologica and member of editorial board of many academic journals.
References
- ↑ Serulle, Y.; Sugimori, M.; Llinas, R. R. (30 January 2007). "Imaging synaptosomal calcium concentration microdomains and vesicle fusion by using total internal reflection fluorescent microscopy". Proceedings of the National Academy of Sciences. 104 (5): 1697–1702. Bibcode:2007PNAS..104.1697S. doi: 10.1073/pnas.0610741104 . PMC 1785242 . PMID 17242349.
- ↑ Llinas, R; Sugimori, M; Silver, R. (1 May 1992). "Microdomains of high calcium concentration in a presynaptic terminal". Science. 256 (5057): 677–679. Bibcode:1992Sci...256..677L. doi:10.1126/science.1350109. PMID 1350109.
- ↑ Demuro, A; Parker, I (Nov–Dec 2006). "Imaging single-channel calcium microdomains". Cell Calcium. 40 (5–6): 413–22. doi:10.1016/j.ceca.2006.08.006. PMC 1694561 . PMID 17067668.
- ↑ Montero M, Brini M, Marsault R, Alvarez J, Sitia R, Pozzan T, Rizzuto R (1995). "Monitoring dynamic changes in free Ca2+ concentration in the endoplasmic reticulum of intact cells". EMBO J. 14 (22): 5467–75. doi:10.1002/j.1460-2075.1995.tb00233.x. PMC 394660 . PMID 8521803.
- ↑ Tian, J.; Xie, Z. J. (August 2007). "The Na-K-ATPase and calcium-signaling microdomains". Physiology. 23 (4): 205–211. doi:10.1152/physiol.00008.2008. PMC 5375021 . PMID 18697994.
- ↑ Lopez-Caamal, F.; Oyarzun, D.A.; Middleton, R.H.; Garcia, M.R. (May 2014). "Spatial Quantification of Cytosolic Ca2+ Accumulation in Nonexcitable Cells:An Analytical Study". IEEE/ACM Transactions on Computational Biology and Bioinformatics. 11 (3): 592–603. doi: 10.1109/TCBB.2014.2316010 . PMID 26356026.
- ↑ Axelrod, D. (2008). Total Internal Reflection Fluorescence Microscopy. In J. J. Correia & H. W. Detrich (Eds.), Biophysical Tools for Biologists, Vol 2: In Vivo Techniques (Vol. 89, pp. 169-221).
- ↑ Bunik, V.; Kaehne, T.; Degtyarev, D.; Shcherbakova, T.; Reiser, G. (2008). "Novel isoenzyme of 2-oxoglutarate dehydrogenase is identified in brain, but not in heart". FEBS Journal. 275 (20): 4990–5006. doi:10.1111/j.1742-4658.2008.06632.x. PMID 18783430. S2CID 21034449.
- ↑ Castillo, K.; Bacigalupo, J.; Restrepo, D. (2008). "Calcium Microdomains in the Chemosensory Cilia of Olfactory Receptor Neurons". Chemical Senses. 33 (8): S61. doi: 10.1093/chemse/bjn065 .
- ↑ Clark, A. J. (2008). "Observation of calcium microdomains at the uropod of living morphologically polarized human neutrophils using flash lamp-based fluorescence microscopy". Cytometry Part A. 73A (7): 673–678. doi:10.1002/cyto.a.20580. PMC 3180874 . PMID 18496849.
- ↑ Francis, A. A.; Mehta, B.; Zenisek, D. (2011). "Development of new peptide-based tools for studying synaptic ribbon function". Journal of Neurophysiology. 106 (2): 1028–1037. doi:10.1152/jn.00255.2011. PMC 3154815 . PMID 21653726.
- ↑ Higgins, E. R. R. (2007). "Modelling calcium microdomains using homogenisation". Journal of Theoretical Biology. 247 (4): 623–644. Bibcode:2007JThBi.247..623H. doi:10.1016/j.jtbi.2007.03.019. PMC 1991275 . PMID 17499276.
- ↑ Ibarretxe, G.; Perrais, D.; Jaskolski, F.; Vimeney, A.; Mulle, C. (2007). "Fast regulation of axonal growth cone motility by electrical activity". Journal of Neuroscience. 27 (29): 7684–7695. doi: 10.1523/jneurosci.1070-07.2007 . PMC 6672867 . PMID 17634363.
- ↑ Lisman, J. E.; Raghavachari, S.; Tsien, R. W. (2007). "The sequence of events that underlie quantal transmission at central glutamatergic synapses". Nature Reviews Neuroscience. 8 (8): 597–609. doi:10.1038/nrn2191. PMID 17637801. S2CID 18365674.
- ↑ Marchaland, J.; Cali, C.; Voglmaier, S. M.; Li, H.; Regazzi, R.; Edwards, R. H.; Bezzi, P. (2009). "Posters". Glia. 57 (13): S45. doi:10.1002/glia.20915. PMC 7165548 .
- ↑ Petibois, C.; Desbat, B. (2010). "Clinical application of FTIR imaging: new reasons for hope". Trends in Biotechnology. 28 (10): 495–500. doi:10.1016/j.tibtech.2010.07.003. PMID 20828847.
- ↑ Ravier, M. A.; Cheng-Xue, R.; Palmer, A. E.; Henquin, J. C.; Gilon, P. (2010). "Subplasmalemmal Ca(2+) measurements in mouse pancreatic beta cells support the existence of an amplifying effect of glucose on insulin secretion". Diabetologia. 53 (9): 1947–1957. doi:10.1007/s00125-010-1775-z. PMC 3297670 . PMID 20461354.
- ↑ Ravier, M. A.; Tsuboi, T.; Rutter, G. A. (2008). "Imaging a target of Ca(2+) signalling: Dense core granule exocytosis viewed by total internal reflection fluorescence microscopy". Methods. 46 (3): 233–238. doi:10.1016/j.ymeth.2008.09.016. PMC 2597054 . PMID 18854212.
- ↑ Shigetomi, E.; Kracun, S.; Khakh, B. S. (2010). "Monitoring astrocyte calcium microdomains with improved membrane targeted GCaMP reporters". Neuron Glia Biology. 6 (3): 183–191. doi:10.1017/s1740925x10000219. PMC 3136572 . PMID 21205365.
- ↑ Thomsen, L. B. T. L. B.; Jorntell, H.; Midtgaard, J. (2010). "Presynaptic calcium signalling in cerebellar mossy fibres". Frontiers in Neural Circuits. 4: 1. doi: 10.3389/neuro.04.001.2010 . PMC 2821199 . PMID 20162034.
- ↑ Zhang, C. F.; Liu, F. C.; Liu, X. B.; Chen, D. J. (2010). "Protective effect of N-acetylcysteine against BDE-209-induced neurotoxicity in primary cultured neonatal rat hippocampal neurons in vitro". International Journal of Developmental Neuroscience. 28 (6): 521–528. doi:10.1016/j.ijdevneu.2010.05.003. PMID 20546880. S2CID 23209232.