Related Research Articles

A spermatozoon is a motile sperm cell, or moving form of the haploid cell that is the male gamete. A spermatozoon joins an ovum to form a zygote.

Fertilisation or fertilization, also known as generative fertilisation, syngamy and impregnation, is the fusion of gametes to give rise to a zygote and initiate its development into a new individual organism or offspring. While processes such as insemination or pollination, which happen before the fusion of gametes, are also sometimes informally referred to as fertilisation, these are technically separate processes. The cycle of fertilisation and development of new individuals is called sexual reproduction. During double fertilisation in angiosperms, the haploid male gamete combines with two haploid polar nuclei to form a triploid primary endosperm nucleus by the process of vegetative fertilisation.

Intracytoplasmic sperm injection is an in vitro fertilization (IVF) procedure in which a single sperm cell is injected directly into the cytoplasm of an egg. This technique is used in order to prepare the gametes for the obtention of embryos that may be transferred to a maternal uterus. With this method, the acrosome reaction is skipped.
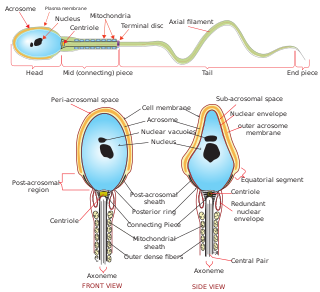
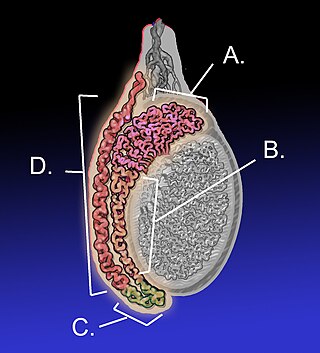
The acrosome is an organelle that develops over the anterior (front) half of the head in the spermatozoa of humans, and many other animals. It is a cap-like structure derived from the Golgi apparatus. In placental mammals, the acrosome contains degradative enzymes. These enzymes break down the outer membrane of the ovum, called the zona pellucida, allowing the haploid nucleus in the sperm cell to join with the haploid nucleus in the ovum. This shedding of the acrosome, or acrosome reaction, can be stimulated in vitro by substances a sperm cell may encounter naturally such as progesterone or follicular fluid, as well as the more commonly used calcium ionophore A23187. This can be done to serve as a positive control when assessing the acrosome reaction of a sperm sample by flow cytometry or fluorescence microscopy. This is usually done after staining with a fluoresceinated lectin such as FITC-PNA, FITC-PSA, FITC-ConA, or fluoresceinated antibody such as FITC-CD46.
The epididymis is an elongated tubular structure attached to the posterior side of each one of the two male reproductive glands, the testicles. It is a single, narrow, tightly coiled tube in adult humans, 6 to 7 centimetres in length; uncoiled the tube would be approximately 6 m long. It connects the testicle to the vas deferens in the male reproductive system. The epididymis serves as an interconnection between the multiple efferent ducts at the rear of a testicle (proximally), and the vas deferens (distally). Its primary function is the storage, maturation and transport of sperm cells.

During fertilization, a sperm must first fuse with the plasma membrane and then penetrate the female egg cell to fertilize it. Fusing to the egg cell usually causes little problem, whereas penetrating through the egg's hard shell or extracellular matrix can be more difficult. Therefore, sperm cells go through a process known as the acrosome reaction, which is the reaction that occurs in the acrosome of the sperm as it approaches the egg.
Hyperactivation is a type of sperm motility. Hyperactivated sperm motility is characterised by a high amplitude, asymmetrical beating pattern of the sperm tail (flagellum). This type of motility may aid in sperm penetration of the zona pellucida, which encloses the ovum.

Acrosin is a digestive enzyme that acts as a protease. In humans, acrosin is encoded by the ACR gene. Acrosin is released from the acrosome of spermatozoa as a consequence of the acrosome reaction. It aids in the penetration of the Zona Pellucida.

Sperm is the male reproductive cell, or gamete, in anisogamous forms of sexual reproduction. Animals produce motile sperm with a tail known as a flagellum, which are known as spermatozoa, while some red algae and fungi produce non-motile sperm cells, known as spermatia. Flowering plants contain non-motile sperm inside pollen, while some more basal plants like ferns and some gymnosperms have motile sperm.

Spermiogenesis is the final stage of spermatogenesis, during which the spermatids develop into mature spermatozoa. At the beginning of the stage, the spermatid is a more or less circular cell containing a nucleus, Golgi apparatus, centriole and mitochondria; by the end of the process, it has radically transformed into an elongated spermatozoon, complete with a head, midpiece, and tail.
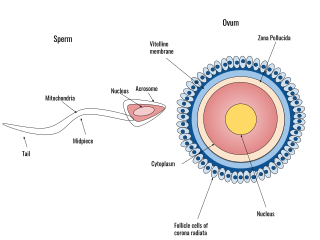
Human fertilization is the union of an egg and sperm, occurring primarily in the ampulla of the fallopian tube. The result of this union leads to the production of a fertilized egg called a zygote, initiating embryonic development. Scientists discovered the dynamics of human fertilization in the 19th century.
Male infertility refers to a sexually mature male's inability to impregnate a fertile female. In humans, it accounts for 40–50% of infertility. It affects approximately 7% of all men. Male infertility is commonly due to deficiencies in the semen, and semen quality is used as a surrogate measure of male fecundity. More recently, advance sperm analyses that examine intracellular sperm components are being developed.
Decapacitation factor (DF) is composed of sperm surface-associated proteins which modulate the fertilizing ability of spermatozoa. Decapacitation is a reversible process that converts fertile, capacitated sperm to less-fertile uncapacitated sperm. This activity is achieved by interaction between cholesterol, phospholipids and fibronectin-like substances and delivered via small vesicles in seminal plasma. DF prevents onset of capacitation. Many DFs are released in secretions from the epididymis and accessory organs of the male reproductive system. However, some DFs have been identified that are located on the acrosome of sperm. Normally, capacitation is initiated through the loss of DF before the spermatozoa can perform the acrosomal reaction. Physiologically decapacitation will inhibit the acrosomal reaction as DFs reassociate onto the sperm surface. For example, one way this can be achieved is through spermatozoal membrane stabilization by maintaining physiological cholesterol/phospholipid ratio.
The hamster zona-free ovum test, or hamster egg-penetration test, or sometimes just hamster test, is an in-vitro test used to study physiological profile of spermatozoa. The primary application of the test is to diagnose male infertility caused by sperm unable to penetrate the ova. The test has limited value, due to expense and a high false negative rate.

A semen analysis, also called seminogram or spermiogram, evaluates certain characteristics of a male's semen and the sperm contained therein. It is done to help evaluate male fertility, whether for those seeking pregnancy or verifying the success of vasectomy. Depending on the measurement method, just a few characteristics may be evaluated or many characteristics may be evaluated. Collection techniques and precise measurement method may influence results.

Sperm motility describes the ability of sperm to move properly through the female reproductive tract or through water to reach the egg. Sperm motility can also be thought of as the quality, which is a factor in successful conception; sperm that do not "swim" properly will not reach the egg in order to fertilize it. Sperm motility in mammals also facilitates the passage of the sperm through the cumulus oophorus and the zona pellucida, which surround the mammalian oocyte.
Sperm sorting is a means of choosing what type of sperm cell is to fertilize the egg cell. Several conventional techniques of centrifugation or swim-up. Newly applied methods such as flow cytometry expand the possibilities of sperm sorting and new techniques of sperm sorting are being developed.
Sperm guidance is the process by which sperm cells (spermatozoa) are directed to the oocyte (egg) for the aim of fertilization. In the case of marine invertebrates the guidance is done by chemotaxis. In the case of mammals, it appears to be done by chemotaxis, thermotaxis and rheotaxis.
Semen cryopreservation is a procedure to preserve sperm cells. Semen can be used successfully indefinitely after cryopreservation. It can be used for sperm donation where the recipient wants the treatment in a different time or place, or as a means of preserving fertility for men undergoing vasectomy or treatments that may compromise their fertility, such as chemotherapy, radiation therapy or surgery. It is also often used by trans women prior to medically transitioning in ways that affect fertility, such as feminizing hormone therapy and orchiectomies.
Antisperm antibodies (ASA) are antibodies produced against sperm antigens.
References
- ↑ Johnson MH (2007). Essential reproduction (6th ed.). Malden Massachusetts: Blackwell Scientific Publications. pp. 177–178. ISBN 978-1-4051-1866-8.
- ↑ Lozano GM, Bejarano I, Espino J, González D, Ortiz A, García JF, Rodríguez AB, Pariente JA (2009). "Density gradient capacitation is the most suitable method to improve fertilization and to reduce DNA fragmentation positive spermatozoa of infertile men". Anatolian Journal of Obstetrics & Gynecology. 3 (1): 1–7.
- ↑ Okabe M (2013). "The cell biology of mammalian fertilization". Development. 140 (22): 4471–4479. doi: 10.1242/dev.090613 . PMID 24194470. S2CID 2015865.
- ↑ Visconti PE, Galantino-Homer H, Moore GD, Bailey JL, Ning X, Fornes M, Kopf GS (1998). "The molecular basis of sperm capacitation". Journal of Andrology. 19 (2): 242–248. doi: 10.1002/j.1939-4640.1998.tb01994.x . PMID 9570749. S2CID 14590131.
- ↑ Puga Molina LC, Luque GM, Balestrini PA, Marín-Briggiler CI, Romarowski A, Buffone MG (2018). "Molecular Basis of Human Sperm Capacitation". Frontiers in Cell and Developmental Biology. 6: 72. doi: 10.3389/fcell.2018.00072 . PMC 6078053 . PMID 30105226.
- ↑ Nabavi N, Todehdehghan F, Shiravi A (September 2013). "Effect of caffeine on motility and vitality of sperm and in vitro fertilization of outbreed mouse in T6 and M16 media". Iranian Journal of Reproductive Medicine. 11 (9): 741–746. PMC 3941327 . PMID 24639814.
- 1 2 Barakat IA, Danfour MA, Galewan FA, Dkhil MA (2015). "Effect of various concentrations of caffeine, pentoxifylline, and kallikrein on hyperactivation of frozen bovine semen". BioMed Research International. 2015: 948575. doi: 10.1155/2015/948575 . PMC 4407405 . PMID 25950005.
- ↑ Parrish JJ, Susko-Parrish J, Winer MA, First NL (1988). "Capacitation of bovine sperm by heparin". Biology of Reproduction. 38 (5): 1171–1180. doi: 10.1095/biolreprod38.5.1171 . PMID 3408784.
- ↑ Way AL, Killian GJ (2002). "Capacitation and induction of the acrosome reaction in bull spermatozoa with norepinephrine". Journal of Andrology. 23 (3): 352–357. doi:10.1002/j.1939-4640.2002.tb02242.x. PMID 12002437.
- ↑ "Sperm Swim up – Percoll | Fertilitycrete".
- 1 2 Mortimer D, Mortimer ST (2013). "Computer-Aided Sperm Analysis (CASA) of sperm motility and hyperactivation". Spermatogenesis. Methods in Molecular Biology. Vol. 927. pp. 77–87. doi:10.1007/978-1-62703-038-0_8. ISBN 978-1-62703-037-3. PMID 22992905.
- ↑ Verstegen J, Iguer-Ouada M, Onclin K (2002). "Computer assisted semen analyzers in andrology research and veterinary practice". Theriogenology. 57 (1): 149–179. doi:10.1016/S0093-691X(01)00664-1. PMID 11775967.
- ↑ Lu HY, Lu JC, Hu YA, Wang YM, Huang YF (2002). "[Detection of human sperm morphology and acrosome reaction with Coomassie brilliant blue staining]". Zhonghua Nan Ke Xue = National Journal of Andrology. 8 (3): 204–206. PMID 12478845.
- ↑ Cheng FP, Fazeli A, Voorhout WF, Marks A, Bevers MM, Colenbrander B (1996). "Use of peanut agglutinin to assess the acrosomal status and the zona pellucida-induced acrosome reaction in stallion spermatozoa". Journal of Andrology. 17 (6): 674–682. doi:10.1002/j.1939-4640.1996.tb01852.x. PMID 9016398.
- ↑ Lybaert P, Danguy A, Leleux F, Meuris S, Lebrun P (2009). "Improved methodology for the detection and quantification of the acrosome reaction in mouse spermatozoa". Histology and Histopathology. 24 (8): 999–1007. doi:10.14670/HH-24.999. PMID 19554507.
- ↑ Ozaki T, Takahashi K, Kanasaki H, Miyazaki K (2002). "Evaluation of acrosome reaction and viability of human sperm with two fluorescent dyes". Archives of Gynecology and Obstetrics. 266 (2): 114–117. doi:10.1007/s004040000112. PMID 12049293. S2CID 19898325.
- ↑ Chang MC (1951). "Fertilizing capacity of spermatozoa deposited into the fallopian tubes". Nature. 168 (4277): 697–698. Bibcode:1951Natur.168..697C. doi:10.1038/168697b0. PMID 14882325. S2CID 4180774.
- ↑ Austin CR (1951). "Observations on the penetration of the sperm in the mammalian egg". Australian Journal of Scientific Research B. 4 (4): 581–596. doi: 10.1071/BI9510581 . PMID 14895481.
- ↑ "Obituary: Colin Austin" (PDF). Australian Academy of Science Newsletter. 60: 11. 2004. Archived from the original (PDF) on 2008-07-19. Retrieved 2008-07-16.