
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration.

Atomic absorption spectroscopy (AAS) and atomic emission spectroscopy (AES) is a spectroanalytical procedure for the quantitative determination of chemical elements by free atoms in the gaseous state. Atomic absorption spectroscopy is based on absorption of light by free metallic ions.

Titration is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte. A reagent, termed the titrant or titrator, is prepared as a standard solution of known concentration and volume. The titrant reacts with a solution of analyte to determine the analyte's concentration. The volume of titrant that reacted with the analyte is termed the titration volume.

The enzyme-linked immunosorbent assay (ELISA) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay uses a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand in a liquid sample using antibodies directed against the protein to be measured. ELISA has been used as a diagnostic tool in medicine, plant pathology, and biotechnology, as well as a quality control check in various industries.
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a mass spectrum, a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures.

An automated analyser is a medical laboratory instrument designed to measure different chemicals and other characteristics in a number of biological samples quickly, with minimal human assistance. These measured properties of blood and other fluids may be useful in the diagnosis of disease.
An assay is an investigative (analytic) procedure in laboratory medicine, mining, pharmacology, environmental biology and molecular biology for qualitatively assessing or quantitatively measuring the presence, amount, or functional activity of a target entity. The measured entity is often called the analyte, the measurand, or the target of the assay. The analyte can be a drug, biochemical substance, chemical element or compound, or cell in an organism or organic sample. An assay usually aims to measure an analyte's intensive property and express it in the relevant measurement unit.

Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte based on its mass. The principle of this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as the relative quantities of the other constituents are known.

Elemental analysis is a process where a sample of some material is analyzed for its elemental and sometimes isotopic composition. Elemental analysis can be qualitative, and it can be quantitative. Elemental analysis falls within the ambit of analytical chemistry, the instruments involved in deciphering the chemical nature of our world.
An immunoassay (IA) is a biochemical test that measures the presence or concentration of a macromolecule or a small molecule in a solution through the use of an antibody (usually) or an antigen (sometimes). The molecule detected by the immunoassay is often referred to as an "analyte" and is in many cases a protein, although it may be other kinds of molecules, of different sizes and types, as long as the proper antibodies that have the required properties for the assay are developed. Analytes in biological liquids such as serum or urine are frequently measured using immunoassays for medical and research purposes.
Microdialysis is a minimally-invasive sampling technique that is used for continuous measurement of free, unbound analyte concentrations in the extracellular fluid of virtually any tissue. Analytes may include endogenous molecules to assess their biochemical functions in the body, or exogenous compounds to determine their distribution within the body. The microdialysis technique requires the insertion of a small microdialysis catheter into the tissue of interest. The microdialysis probe is designed to mimic a blood capillary and consists of a shaft with a semipermeable hollow fiber membrane at its tip, which is connected to inlet and outlet tubing. The probe is continuously perfused with an aqueous solution (perfusate) that closely resembles the (ionic) composition of the surrounding tissue fluid at a low flow rate of approximately 0.1-5μL/min. Once inserted into the tissue or (body)fluid of interest, small solutes can cross the semipermeable membrane by passive diffusion. The direction of the analyte flow is determined by the respective concentration gradient and allows the usage of microdialysis probes as sampling as well as delivery tools. The solution leaving the probe (dialysate) is collected at certain time intervals for analysis.

The AutoAnalyzer is an automated analyzer using a flow technique called continuous flow analysis (CFA), or more correctly segmented flow analysis (SFA) first made by the Technicon Corporation. The instrument was invented in 1957 by Leonard Skeggs, PhD and commercialized by Jack Whitehead's Technicon Corporation. The first applications were for clinical analysis, but methods for industrial and environmental analysis soon followed. The design is based on segmenting a continuously flowing stream with air bubbles.

Fast atom bombardment (FAB) is an ionization technique used in mass spectrometry in which a beam of high energy atoms strikes a surface to create ions. It was developed by Michael Barber at the University of Manchester in 1980. When a beam of high energy ions is used instead of atoms, the method is known as liquid secondary ion mass spectrometry (LSIMS). In FAB and LSIMS, the material to be analyzed is mixed with a non-volatile chemical protection environment, called a matrix, and is bombarded under vacuum with a high energy beam of atoms. The atoms are typically from an inert gas such as argon or xenon. Common matrices include glycerol, thioglycerol, 3-nitrobenzyl alcohol (3-NBA), 18-crown-6 ether, 2-nitrophenyloctyl ether, sulfolane, diethanolamine, and triethanolamine. This technique is similar to secondary ion mass spectrometry and plasma desorption mass spectrometry.
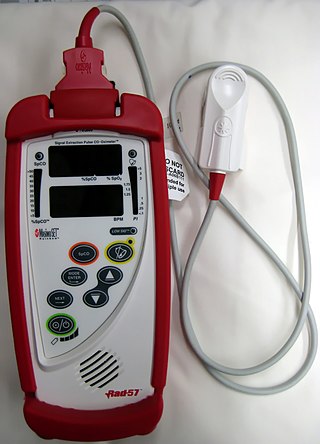
A CO-oximeter is a device that measures the oxygen carrying state of hemoglobin in a blood specimen, including oxygen-carrying hemoglobin (O2Hb), non-oxygen-carrying but normal hemoglobin (HHb), as well as the dyshemoglobins such as carboxyhemoglobin (COHb) and methemoglobin (MetHb). The use of 'CO' rather than 'Co' or 'co' is more appropriate since this designation represents a device that measures carbon monoxide (CO) bound to hemoglobin, as distinguished from simple oximetry which measures hemoglobin bound to molecular oxygen—O2Hb—or hemoglobin capable of binding to molecular oxygen—HHb. Simpler oximeters may report oxygen saturation alone, i.e. the ratio of oxyhemoglobin to total 'bindable' hemoglobin. CO-oximetry is useful in defining the causes for hypoxemia, or hypoxia,.
Surface-enhanced laser desorption/ionization (SELDI) is a soft ionization method in mass spectrometry (MS) used for the analysis of protein mixtures. It is a variation of matrix-assisted laser desorption/ionization (MALDI). In MALDI, the sample is mixed with a matrix material and applied to a metal plate before irradiation by a laser, whereas in SELDI, proteins of interest in a sample become bound to a surface before MS analysis. The sample surface is a key component in the purification, desorption, and ionization of the sample. SELDI is typically used with time-of-flight (TOF) mass spectrometers and is used to detect proteins in tissue samples, blood, urine, or other clinical samples, however, SELDI technology can potentially be used in any application by simply modifying the sample surface.

A medical laboratory or clinical laboratory is a laboratory where tests are conducted out on clinical specimens to obtain information about the health of a patient to aid in diagnosis, treatment, and prevention of disease. Clinical Medical laboratories are an example of applied science, as opposed to research laboratories that focus on basic science, such as found in some academic institutions.
Flow injection analysis (FIA) is an approach to chemical analysis. It is accomplished by injecting a plug of sample into a flowing carrier stream. The principle is similar to that of Segmented Flow Analysis (SFA) but no air is injected into the sample or reagent streams..
Sample preparation for mass spectrometry is used for the optimization of a sample for analysis in a mass spectrometer (MS). Each ionization method has certain factors that must be considered for that method to be successful, such as volume, concentration, sample phase, and composition of the analyte solution. Quite possibly the most important consideration in sample preparation is knowing what phase the sample must be in for analysis to be successful. In some cases the analyte itself must be purified before entering the ion source. In other situations, the matrix, or everything in the solution surrounding the analyte, is the most important factor to consider and adjust. Often, sample preparation itself for mass spectrometry can be avoided by coupling mass spectrometry to a chromatography method, or some other form of separation before entering the mass spectrometer. In some cases, the analyte itself must be adjusted so that analysis is possible, such as in protein mass spectrometry, where usually the protein of interest is cleaved into peptides before analysis, either by in-gel digestion or by proteolysis in solution.

Additional information needed
Max Davis Liston is an American pioneer in the development of instruments for infrared spectrophotometry and non-dispersive infrared analysis. Two of his innovations, the breaker-type direct-coupled amplifier and the vacuum thermocouple, were essential to the development of infrared spectrometry technology. Among others, Liston has developed instruments for capnometry, the measurement of carbon dioxide in respiratory gases, used to monitor patients. He also developed instruments to measure smog and car exhaust emissions, essential to attempts to improve Los Angeles air quality in the 1950s.














