
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen or sulfur. Rarer still, they may contain elements such as phosphorus, chlorine, and bromine.

Apocynaceae is a family of flowering plants that includes trees, shrubs, herbs, stem succulents, and vines, commonly known as the dogbane family, because some taxa were used as dog poison. Members of the family are native to the European, Asian, African, Australian, and American tropics or subtropics, with some temperate members. The former family Asclepiadaceae is considered a subfamily of Apocynaceae and contains 348 genera. A list of Apocynaceae genera may be found here.

Catharanthus is a genus of flowering plants in the family Apocynaceae. Like the genus Vinca, they are known commonly as periwinkles. There are eight known species. Seven are endemic to Madagascar, though one, C. roseus, is widely naturalized around the world. The eighth species, C. pusillus, is native to India and Sri Lanka. The name Catharanthus comes from the Greek for "pure flower".
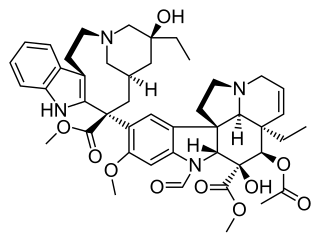
Vincristine, also known as leurocristine and marketed under the brand name Oncovin among others, is a chemotherapy medication used to treat a number of types of cancer. This includes acute lymphocytic leukemia, acute myeloid leukemia, Hodgkin's disease, neuroblastoma, and small cell lung cancer among others. It is given intravenously.

Vinca is a genus of flowering plants in the family Apocynaceae, native to Europe, northwest Africa and southwest Asia. The English name periwinkle is shared with the related genus Catharanthus.

Fort-Dauphin is a city on the southeast coast of Madagascar. It is the capital of the Anosy Region and of the Taolagnaro District. It has been a port of local importance since the early 1500s. A new port, the Ehoala Port was built in 2006–2009. Fort-Dauphin was the first French settlement in Madagascar.

Vinorelbine (NVB), sold under the brand name Navelbine among others, is a chemotherapy medication used to treat a number of types of cancer. This includes breast cancer and non-small cell lung cancer. It is given by injection into a vein or by mouth.

Vinblastine (VBL), sold under the brand name Velban among others, is a chemotherapy medication, typically used with other medications, to treat a number of types of cancer. This includes Hodgkin's lymphoma, non-small-cell lung cancer, bladder cancer, brain cancer, melanoma, and testicular cancer. It is given by injection into a vein.

Vinca alkaloids are a set of anti-mitotic and anti-microtubule alkaloid agents originally derived from the periwinkle plant Catharanthus roseus and other vinca plants. They block beta-tubulin polymerization in a dividing cell.

Vindesine, also termed Eldisine, is a semisynthetic vinca alkaloid derived from the flowering plant Catharanthus roseus. Like the natural and semisynthetic vinca alkaloids derived from this plant, vindesine is an inhibitor of mitosis that is used as a chemotherapy drug. By inhibiting mitosis, vinedsine blocks the proliferation of cells, particularly the rapidly proliferation cells of certain types of cancer. It is used, generally in combination with other chemotherapeutic drugs, in the treatment of various malignancies such as leukaemia, lymphoma, melanoma, breast cancer, and lung cancer.
Ambovombe-Androy, or just Ambovombe, is a city in the far south of Madagascar, and the capital of the Androy region. Ambovombe has now acquired city status with an officially estimated population in 2018 of 114,230.

A mitotic inhibitor, microtubule inhibitor, or tubulin inhibitor, is a drug that inhibits mitosis, or cell division, and is used in treating cancer, gout, and nail fungus. These drugs disrupt microtubules, which are structures that pull the chromosomes apart when a cell divides. Mitotic inhibitors are used in cancer treatment, because cancer cells are able to grow through continuous division that eventually spread through the body (metastasize). Thus, cancer cells are more sensitive to inhibition of mitosis than normal cells. Mitotic inhibitors are also used in cytogenetics, where they stop cell division at a stage where chromosomes can be easily examined.
Strictosidine synthase (EC 4.3.3.2) is an enzyme in alkaloid biosynthesis that catalyses the condensation of tryptamine with secologanin to form strictosidine in a formal Pictet–Spengler reaction:

Catharanthus trichophyllus is a species of flowering plant in the family Apocynaceae. It is endemic to Madagascar, where it is most common in northern regions.

Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.
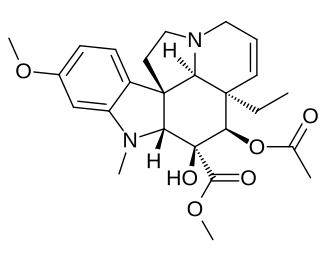
Vindoline is a chemical precursor to vinblastine. Vindoline is formed through biosynthesis from Tabersonine.

Strictosidine is a natural chemical compound and is classified as a glucoalkaloid and a vinca alkaloid. It is formed by the Pictet–Spengler condensation reaction of tryptamine with secologanin, catalyzed by the enzyme strictosidine synthase. Thousands of strictosidine derivatives are sometimes referred to by the broad phrase of monoterpene indole alkaloids. Strictosidine is an intermediate in the biosynthesis of numerous pharmaceutically valuable metabolites including quinine, camptothecin, ajmalicine, serpentine, vinblastine, vincristine and mitragynine.

16-Hydroxytabersonine is a terpene indole alkaloid produced by the plant Catharanthus roseus. The metabolite is an intermediate in the formation of vindoline, a precursor needed for formation of the pharmaceutically valuable vinblastine and vincristine. 16-hydroxytabersonine is formed from the hydroxylation of tabersonine by tabersonine 16-hydroxylase (T16H). Tabersonine 16-O-methyltransferase (16OMT) methylates the hydroxylated 16 position to form 16-methoxytabersonine.

Bis(cyclopentadienyl)titanium(III) chloride, also known as the Nugent–RajanBabu reagent, is the organotitanium compound which exists as a dimer with the formula [(C5H5)2TiCl]2. It is an air sensitive green solid. The complex finds specialized use in synthetic organic chemistry as a single electron reductant.

Catharanthine and vindoline are terpenoid indole alkaloids naturally produced within the Madagascar periwinkle plant whose dimerization produces the anti-cancer drugs vinblastine and vincristine. The precursor of catharanthine and vindoline is strictosidine, the common precursor of all indole alkaloids. The localization of catharanthine and vindoline within the plant tissue has been heavily studied in recent years with conflicting results. The dimerization of catharanthine and vindoline to form vinblastine and vincristine is catalyzed by a peroxidase and a reductase, and includes several intermediate compounds.
















































