Agent Orange is a chemical herbicide and defoliant, one of the tactical use Rainbow Herbicides.

Polychlorinated biphenyls (PCBs) are highly carcinogenic chemical compounds, formerly used in industrial and consumer products, whose production was banned in the United States by the Toxic Substances Control Act in 1976 and internationally by the Stockholm Convention on Persistent Organic Pollutants in 2001.
Polychlorinated dibenzodioxins (PCDDs), or simply dioxins, are a group of long-lived polyhalogenated organic compounds that are primarily anthropogenic, and contribute toxic, persistent organic pollution in the environment.

The Seveso disaster was an industrial accident that occurred around 12:37 pm on 10 July 1976, in a small chemical manufacturing plant approximately 20 kilometres (12 mi) north of Milan in the Lombardy region of Italy. It resulted in the highest known exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in residential populations, which gave rise to numerous scientific studies and standardized industrial safety regulations, including the European Union's Seveso III Directive. This accident was ranked eighth in a list of the worst man-made environmental disasters by Time magazine in 2010.

Rosacea is a long-term skin condition that typically affects the face. It results in redness, pimples, swelling, and small and superficial dilated blood vessels. Often, the nose, cheeks, forehead, and chin are most involved. A red, enlarged nose may occur in severe disease, a condition known as rhinophyma.

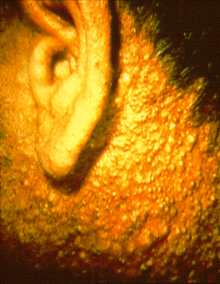
2,4,5-Trichlorophenoxyacetic acid, a synthetic auxin, is a chlorophenoxy acetic acid herbicide used to defoliate broad-leafed plants. It was developed in the late 1940s, synthesized by reaction of 2,4,5-Trichlorophenol and chloroacetic acid. It was widely used in the agricultural industry until being phased out, starting in the late 1970s due to toxicity concerns. Agent Orange, a defoliant used by the British in the Malayan Emergency and the U.S. in the Vietnam War, was equal parts 2,4,5-T and 2,4-D. 2,4,5-T itself is toxic with a NOAEL of 3 mg/kg/day and a LOAEL of 10 mg/kg/day. Agent Pink contained 100% 2,4,5-T. Additionally, the manufacturing process for 2,4,5-T contaminates this chemical with trace amounts of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). TCDD is a carcinogenic persistent organic pollutant with long-term effects on the environment. With proper temperature control during production of 2,4,5-T, TCDD levels can be held to about .005 ppm. Before the TCDD risk was well understood, early production facilities lacked proper temperature controls and individual batches tested later were found to have as much as 60 ppm of TCDD.
Acne is acneiform eruptions. It is usually used as a synonym for acne vulgaris, but may also refer to:
A toxic tort claim is a specific type of personal injury lawsuit in which the plaintiff claims that exposure to a chemical or dangerous substance caused the plaintiff's injury or disease.

Yushō disease was a mass poisoning by polychlorinated biphenyls (PCBs) which occurred in northern Kyūshū, Japan, in 1968. In January 1968, rice bran oil produced by Kanemi Company in Kyushu was contaminated with PCBs and polychlorinated dibenzofurans (PCDFs) during production. For deodorization, the oil was heated using PCB as the heating medium, circulating through pipes. Due to holes in the pipes the PCB leaked into the rice bran oil. The contaminated rice bran oil was then sold to poultry farmers for use as a feed supplement and to consumers for use in cooking. In February to March 1968, farmers started reporting that their poultry were dying due to apparent difficulty in breathing; altogether 400,000 birds died. About 14,000 people who had consumed the contaminated rice oil were affected in Japan. More than 500 died. Common symptoms included dermal and ocular lesions, and a lowered immune response. Other symptoms included fatigue, headache, cough, and unusual skin sores. Additionally, in children, there were reports of poor cognitive development.

1,4-Dioxin (also referred as dioxin or p-dioxin) is a heterocyclic, organic, non-aromatic compound with the chemical formula C4H4O2. There is an isomeric form of 1,4-dioxin, 1,2-dioxin (or o-dioxin). 1,2-Dioxin is very unstable due to its peroxide-like characteristics.

Polychlorinated dibenzofurans (PCDFs) are a family of organic compounds with one or several of the hydrogens in the dibenzofuran structure replaced by chlorines. For example, 2,3,7,8-tetrachlorodibenzofuran (TCDF) has chlorine atoms substituted for each of the hydrogens on the number 2, 3, 7, and 8 carbons. Polychlorinated dibenzofurans with chlorines at least in positions 2,3,7 and 8 are much more toxic than the parent compound dibenzofurane, with properties and chemical structures similar to polychlorinated dibenzodioxins. These groups together are often inaccurately called dioxins. They are known developmental toxicants, and suspected human carcinogens. PCDFs tend to co-occur with polychlorinated dibenzodioxins (PCDDs). PCDFs can be formed by pyrolysis or incineration at temperatures below 1200 °C of chlorine containing products, such as PVC, PCBs, and other organochlorides, or of non-chlorine containing products in the presence of chlorine donors. Dibenzofurans are known persistent organic pollutants (POP), classified among the dirty dozen in the Stockholm Convention on Persistent Organic Pollutants.

Acneiform eruptions, or acne mimicking eruptions, are a group of skin conditions characterized by small bumps resembling acne. Typically, these bumps are mostly of similar size. Some bumps may be bigger or contain fluid. Generally, blackheads and whiteheads are absent. It tends to appear suddenly, with the chest and back most frequently affected.

Dioxins and dioxin-like compounds (DLCs) are a group of chemical compounds that are persistent organic pollutants (POPs) in the environment. They are mostly by-products of burning or various industrial processes or, in the case of dioxin-like PCBs and PBBs, unwanted minor components of intentionally produced mixtures.
Acne necrotica presents with a primary lesion that is a pruritic or painful erythematous follicular-based papule that develops central necrosis and crusting and heals with a varioliform scar.

2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a polychlorinated dibenzo-p-dioxin (sometimes shortened, though inaccurately, to simply 'dioxin') with the chemical formula C12H4Cl4O2. Pure TCDD is a colorless solid with no distinguishable odor at room temperature. It is usually formed as an unwanted product in burning processes of organic materials or as a side product in organic synthesis.

Holmesburg Prison, given the nickname "The Terrordome," was a prison operated by the city of Philadelphia, Pennsylvania and the Pennsylvania Department of Prisons (PDP) from 1896 to 1995. The facility is located at 8215 Torresdale Ave in the Holmesburg section of Philadelphia. It was decommissioned in 1995 when it closed. As of today, the structure still stands and is occasionally used for prisoner overflow and work programs.
Toxic equivalency factor (TEF) expresses the toxicity of dioxins, furans and PCBs in terms of the most toxic form of dioxin, 2,3,7,8-TCDD. The toxicity of the individual congeners may vary by orders of magnitude.

The Life on the Earth - The Summer of Dioxin, also known as Tracing the Gray Summer, is a 2001 Japanese anime drama film by Satoshi Dezaki, a joint production between the Magic Bus and the GoGo Visual Planning which recounts the true story of the Seveso disaster, a chemical incident occurred over the Italian town of Seveso in 1976, which is still considered one of the worst ecological disasters in history.

1,2,3,4,6,7,8-Heptachlorodibenzo-para-dioxin (often referred to as 1,2,3,4,6,7,8-HpCDD) is a polychlorinated derivative of dibenzo-p-dioxin and can therefore be categorized as polychlorinated dibenzo-p-dioxin (PCDD), a subclass of dioxins which includes 75 congeners. HpCDD is the dibenzo-p-dioxin which is chlorinated at positions 1, 2, 3, 4, 6, 7, and 8. It is a polycyclic heterocyclic organic compound, since HpCDD contains multiple cyclic structures (two benzene rings connected by a 1,4-dioxin ring) in which two different elements (carbon and oxygen) are members of its rings. HpCDD has molecular formula C12HCl7O2 and is an off-white powder, which is insoluble in water.

2,2',3,3',4,4'-Hexachlorobiphenyl is an organic chemical and belongs to a group of compounds called polychlorinated biphenyls. This group of organic compounds was used in transformers as dielectric fluids, until production was banned in 1979. While only being a part of this mixture, it is sometimes referred to as Aroclor 1260.