
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and propane.
Isobutane, also known as i-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH3)3. It is an isomer of butane. Isobutane is a colorless, odorless gas. It is the simplest alkane with a tertiary carbon atom. Isobutane is used as a precursor molecule in the petrochemical industry, for example in the synthesis of isooctane.
Freon is a registered trademark of the Chemours Company and generic descriptor for a number of halocarbon products. They are stable, nonflammable, low toxicity gases or liquids which have generally been used as refrigerants and as aerosol propellants. These include chlorofluorocarbons and hydrofluorocarbons, both of which cause ozone depletion and contribute to global warming. 'Freon' is the brand name for the refrigerants R-12, R-13B1, R-22, R-410A, R-502, and R-503 manufactured by The Chemours Company, and so is not used to label all refrigerants of this type. They emit a strong smell similar to acetone. Freon has been found to cause damage to human health when inhaled in large amounts. Studies have been conducted in the pursuit to find beneficial reuses for gases under the Freon umbrella as an alternative to disposal of the gas.

A refrigerant is a working fluid used in the refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are heavily regulated due to their toxicity, flammability and the contribution of CFC and HCFC refrigerants to ozone depletion and that of HFC refrigerants to climate change.
Difluoromethane, also called difluoromethylene, HFC-32Methylene Fluoride or R-32, is an organic compound of the dihalogenoalkane variety. It has the formula of CH2F2. It is a colorless gas in the ambient atmosphere and is slightly soluble in water, with a high thermal stability. Due to the low melting and boiling point, (-136.0 °C and -51.6 °C respectively) contact with this compound may result in frostbite. In the United States, the Clean Air Act Section 111 on Volatile Organic Compounds (VOC) has listed difluoromethane as an exception (since 1997) from the definition of VOC due to its low production of tropospheric ozone. Difluoromethane is commonly used in endothermic processes such as refrigeration or air conditioning.
Propane refrigeration is a type of compression refrigeration. Propane (R290) has been used successfully in industrial refrigeration for many years, and is emerging as an increasingly viable alternative for homes and businesses. Propane's operating pressures and temperatures are well suited for use in air conditioning equipment, but because of propane’s flammability, great care is required in the manufacture, installation and servicing of equipment that uses it as a refrigerant.
R-410A, sold under the trademarked names AZ-20, EcoFluor R410, Forane 410A, Genetron R410A, Puron, and Suva 410A, is a zeotropic but near-azeotropic mixture of difluoromethane (CH2F2, called R-32) and pentafluoroethane (CHF2CF3, called R-125) that is used as a refrigerant in air conditioning and heat pump applications. R-410A cylinders were colored rose but are no longer specially color-coded, now bearing a standard light gray color.

An icemaker, ice generator, or ice machine may refer to either a consumer device for making ice, found inside a home freezer; a stand-alone appliance for making ice, or an industrial machine for making ice on a large scale. The term "ice machine" usually refers to the stand-alone appliance.

Vapour-compression refrigeration or vapor-compression refrigeration system (VCRS), in which the refrigerant undergoes phase changes, is one of the many refrigeration cycles and is the most widely used method for air conditioning of buildings and automobiles. It is also used in domestic and commercial refrigerators, large-scale warehouses for chilled or frozen storage of foods and meats, refrigerated trucks and railroad cars, and a host of other commercial and industrial services. Oil refineries, petrochemical and chemical processing plants, and natural gas processing plants are among the many types of industrial plants that often utilize large vapor-compression refrigeration systems. Cascade refrigeration systems may also be implemented using two compressors.
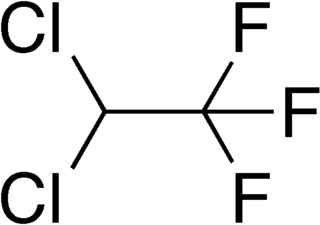
2,2-Dichloro-1,1,1-trifluoroethane or HCFC-123 is considered as an alternative to CFC-11 in low pressure refrigeration and HVAC systems, and should not be used in foam blowing processes or solvent applications. It is also the primary component of the Halotron I fire-extinguishing mixture.

An air source heat pump (ASHP) is a heat pump that can absorb heat from air outside a building and release it inside; it uses the same vapor-compression refrigeration process and much the same equipment as an air conditioner, but in the opposite direction. ASHPs are the most common type of heat pump and, usually being smaller, tend to be used to heat individual houses or flats rather than blocks, districts or industrial processes.
Natural refrigerants are considered substances that serve as refrigerants in refrigeration systems. They are alternatives to synthetic refrigerants such as chlorofluorocarbon (CFC), hydrochlorofluorocarbon (HCFC), and hydrofluorocarbon (HFC) based refrigerants. Unlike other refrigerants, natural refrigerants can be found in nature and are commercially available thanks to physical industrial processes like fractional distillation, chemical reactions such as Haber process and spin-off gases. The most prominent of these include various natural hydrocarbons, carbon dioxide, ammonia, and water. Natural refrigerants are preferred actually in new equipment to their synthetic counterparts for their presumption of higher degrees of sustainability. With the current technologies available, almost 75 percent of the refrigeration and air conditioning sector has the potential to be converted to natural refrigerants.
Refrigerant reclamation is the act of processing used refrigerant gas which has previously been used in some type of refrigeration loop such that it meets specifications for new refrigerant gas. In the United States, the Section 608 of the Clean Air Act of 1990 requires that used refrigerant be processed by a certified reclaimer, which must be licensed by the United States Environmental Protection Agency (EPA), and the material must be recovered and delivered to the reclaimer by EPA-certified technicians.

1,1-Dichloro-1-fluoroethane is a haloalkane with the formula C
2H
3Cl
2F. It is one of the three isomers of dichlorofluoroethane. It belongs to the hydrochlorofluorocarbon (HCFC) family of man-made compounds that contribute significantly to both ozone depletion and global warming when released into the environment.

1-Chloro-1,1-difluoroethane (HCFC-142b) is a haloalkane with the chemical formula CH3CClF2. It belongs to the hydrochlorofluorocarbon (HCFC) family of man-made compounds that contribute significantly to both ozone depletion and global warming when released into the environment. It is primarily used as a refrigerant where it is also known as R-142b and by trade names including Freon-142b.

Hydrofluoroolefins (HFOs) are unsaturated organic compounds composed of hydrogen, fluorine and carbon. These organofluorine compounds are of interest as refrigerants. Unlike traditional hydrofluorocarbons (HFCs) and chlorofluorocarbons (CFCs), which are saturated, HFOs are olefins, otherwise known as alkenes.
Fluorinated gases (F-gases) are a group of gases containing fluorine. They are divided into several types, the main of those are hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), sulphur hexafluoride (SF6). They are used in refrigeration, air conditioning, heat pumps, fire suppression, electronics, aerospace, magnesium industry, foam and high voltage switchgear. As they are greenhouse gases with a strong global warming potential, their use is regulated.
Life Cycle Climate Performance (LCCP) is an evolving method to evaluate the carbon footprint and global warming impact of heating, ventilation, air conditioning (AC), refrigeration systems, and potentially other applications such as thermal insulating foam. It is calculated as the sum of direct, indirect, and embodied greenhouse gas (GHG) emissions generated over the lifetime of the system “from cradle to grave,” i.e. from manufacture to disposal. Direct emissions include all climate forcing effects from the release of refrigerants into the atmosphere, including annual leakage and losses during service and disposal of the unit. Indirect emissions include the climate forcing effects of GHG emissions from the electricity powering the equipment. The embodied emissions include the climate forcing effects of the manufacturing processes, transport, and installation for the refrigerant, materials, and equipment, and for recycle or other disposal of the product at end of its useful life.
The Significant New Alternatives Policy is a program of the EPA to determine acceptable chemical substitutes, and establish which are prohibited or regulated by the EPA. It also establishes a program by which new alternatives may be accepted, and promulgates timelines to the industry regarding phase-outs of substitutes.





![Shipping container for the gas in Japan. Container [( 2%3FT%3F )] GRPU 918195(3)---No,2 [( Pictures taken in Japan )] .jpg](http://upload.wikimedia.org/wikipedia/commons/thumb/8/8b/Container_%E3%80%90_2%3FT%3F_%E3%80%91_GRPU_918195%283%29---No%2C2_%E3%80%90_Pictures_taken_in_Japan_%E3%80%91.jpg/220px-Container_%E3%80%90_2%3FT%3F_%E3%80%91_GRPU_918195%283%29---No%2C2_%E3%80%90_Pictures_taken_in_Japan_%E3%80%91.jpg)










