In chemistry, triiodide usually refers to the triiodide ion, I−
3. This anion, one of the polyhalogen ions, is composed of three iodine atoms. It is formed by combining aqueous solutions of iodide salts and iodine. Some salts of the anion have been isolated, including thallium(I) triiodide (Tl+[I3]−) and ammonium triiodide ([NH4]+[I3]−). Triiodide is observed to be a red colour in solution.

Aluminium iodide is a chemical compound containing aluminium and iodine. Invariably, the name refers to a compound of the composition AlI
3, formed by the reaction of aluminium and iodine or the action of HI on Al metal. The hexahydrate is obtained from a reaction between metallic aluminum or aluminum hydroxide with hydrogen iodide or hydroiodic acid. Like the related chloride and bromide, AlI
3 is a strong Lewis acid and will absorb water from the atmosphere. It is employed as a reagent for the scission of certain kinds of C-O and N-O bonds. It cleaves aryl ethers and deoxygenates epoxides.
Terbium(III) iodide (TbI3) is an inorganic chemical compound.

Scandium triiodide, also known as scandium iodide, is an inorganic compound with the formula ScI3 and is classified as a lanthanide iodide. This salt is a yellowish powder. It is used in metal halide lamps together with similar compounds, such as caesium iodide, because of their ability to maximize emission of UV and to prolong bulb life. The maximized UV emission can be tuned to a range that can initiate photopolymerizations.
Americium(III) iodide or americium triiodide is the chemical compound composed of americium and iodine with the formula AmI3.
Cerium(III) iodide (CeI3) is the compound formed by cerium(III) cations and iodide anions.

Rhodium(III) iodide is an inorganic compound with the formula RhI3. It is a black solid.

Indium(III) iodide or indium triiodide is a chemical compound of indium and iodine with the formula InI3.
Neodymium(III) iodide is an inorganic salt of iodine and neodymium with the formula NdI3. Neodymium uses the +3 oxidation state in the compound. The anhydrous compound is a green powdery solid at room temperature.

Praseodymium(III) iodide is an inorganic salt, consisting of the rare-earth metal praseodymium with hydrogen iodide with the chemical formula PrI3. It forms green crystals. It is soluble in water.

Zirconium(III) iodide is an inorganic compound with the formula ZrI3.

Lanthanum(III) iodide is an inorganic compound containing lanthanum and iodine with the chemical formula LaI
3.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.

Holmium(III) iodide is an iodide of holmium, with the chemical formula of HoI3. It is used as a component of metal halide lamps.
Lutetium compounds are compounds formed by the lanthanide metal lutetium (Lu). In these compounds, lutetium generally exhibits the +3 oxidation state, such as LuCl3, Lu2O3 and Lu2(SO4)3. Aqueous solutions of most lutetium salts are colorless and form white crystalline solids upon drying, with the common exception of the iodide. The soluble salts, such as nitrate, sulfate and acetate form hydrates upon crystallization. The oxide, hydroxide, fluoride, carbonate, phosphate and oxalate are insoluble in water.

Hafnium(III) iodide is an inorganic compound of hafnium and iodine with the formula Hf I3. It is a black solid.
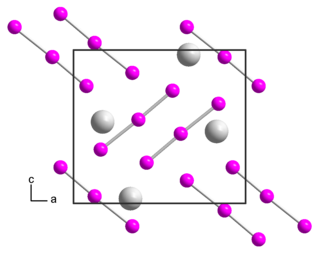
Rubidium triiodide is an inorganic compound with the chemical formula RbI3. It is composed of Rb+ and I−
3.

Curium(III) iodide is the chemical compound with the formula CmI3. Since all isotopes of curium are only artificially produced, the compound has no natural occurrence.