Related Research Articles

Radiation therapy or radiotherapy, often abbreviated RT, RTx, or XRT, is a treatment using ionizing radiation, generally provided as part of cancer therapy to either kill or control the growth of malignant cells. It is normally delivered by a linear particle accelerator. Radiation therapy may be curative in a number of types of cancer if they are localized to one area of the body, and have not spread to other parts. It may also be used as part of adjuvant therapy, to prevent tumor recurrence after surgery to remove a primary malignant tumor. Radiation therapy is synergistic with chemotherapy, and has been used before, during, and after chemotherapy in susceptible cancers. The subspecialty of oncology concerned with radiotherapy is called radiation oncology. A physician who practices in this subspecialty is a radiation oncologist.

Acute radiation syndrome (ARS), also known as radiation sickness or radiation poisoning, is a collection of health effects that are caused by being exposed to high amounts of ionizing radiation in a short period of time. Symptoms can start within an hour of exposure, and can last for several months. Early symptoms are usually nausea, vomiting and loss of appetite. In the following hours or weeks, initial symptoms may appear to improve, before the development of additional symptoms, after which either recovery or death follow.
The therapeutic index is a quantitative measurement of the relative safety of a drug. It is a comparison of the amount of a therapeutic agent that causes the therapeutic effect to the amount that causes toxicity. The related terms therapeutic window or safety window refer to a range of doses optimized between efficacy and toxicity, achieving the greatest therapeutic benefit without resulting in unacceptable side-effects or toxicity.

In medicine, proton therapy, or proton radiotherapy, is a type of particle therapy that uses a beam of protons to irradiate diseased tissue, most often to treat cancer. The chief advantage of proton therapy over other types of external beam radiotherapy is that the dose of protons is deposited over a narrow range of depth; hence in minimal entry, exit, or scattered radiation dose to healthy nearby tissues.

Radiosurgery is surgery using radiation, that is, the destruction of precisely selected areas of tissue using ionizing radiation rather than excision with a blade. Like other forms of radiation therapy, it is usually used to treat cancer. Radiosurgery was originally defined by the Swedish neurosurgeon Lars Leksell as "a single high dose fraction of radiation, stereotactically directed to an intracranial region of interest".
Radioresistance is the level of ionizing radiation that organisms are able to withstand.
Radiosensitivity is the relative susceptibility of cells, tissues, organs or organisms to the harmful effect of ionizing radiation.
Dose fractionation effects are utilised in the treatment of cancer with radiation therapy. When the total dose of radiation is divided into several, smaller doses over a period of several days, there are fewer toxic effects on healthy cells. This maximizes the effect of radiation on cancer and minimizes the negative side effects. A typical fractionation scheme divides the dose into 30 units delivered every weekday over six weeks.
The radiation-induced bystander effect is the phenomenon in which unirradiated cells exhibit irradiated effects as a result of signals received from nearby irradiated cells. In November 1992, Hatsumi Nagasawa and John B. Little first reported this radiobiological phenomenon.
Particle therapy is a form of external beam radiotherapy using beams of energetic neutrons, protons, or other heavier positive ions for cancer treatment. The most common type of particle therapy as of August 2021 is proton therapy.
Radiobiology is a field of clinical and basic medical sciences that involves the study of the effects of ionizing radiation on living things, in particular health effects of radiation. Ionizing radiation is generally harmful and potentially lethal to living things but can have health benefits in radiation therapy for the treatment of cancer and thyrotoxicosis. Its most common impact is the induction of cancer with a latent period of years or decades after exposure. High doses can cause visually dramatic radiation burns, and/or rapid fatality through acute radiation syndrome. Controlled doses are used for medical imaging and radiotherapy.
In radiobiology, the relative biological effectiveness is the ratio of biological effectiveness of one type of ionizing radiation relative to another, given the same amount of absorbed energy. The RBE is an empirical value that varies depending on the type of ionizing radiation, the energies involved, the biological effects being considered such as cell death, and the oxygen tension of the tissues or so-called oxygen effect.

Follicular dendritic cell sarcoma (FDCS) is an extremely rare neoplasm. While the existence of FDC tumors was predicted by Lennert in 1978, the tumor wasn't fully recognized as its own cancer until 1986 after characterization by Monda et al. It accounts for only 0.4% of soft tissue sarcomas, but has significant recurrent and metastatic potential and is considered an intermediate grade malignancy. The major hurdle in treating FDCS has been misdiagnosis. It is a newly characterized cancer, and because of its similarities in presentation and markers to lymphoma, both Hodgkin and Non-Hodgkin subtypes, diagnosis of FDCS can be difficult. With recent advancements in cancer biology better diagnostic assays and chemotherapeutic agents have been made to more accurately diagnose and treat FDCS.
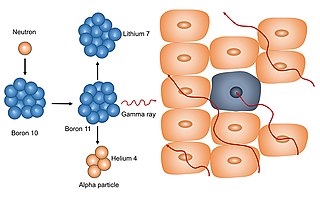
Neutron capture therapy (NCT) is a type of radiotherapy for treating locally invasive malignant tumors such as primary brain tumors, recurrent cancers of the head and neck region, and cutaneous and extracutaneous melanomas. It is a two-step process: first, the patient is injected with a tumor-localizing drug containing the stable isotope boron-10 (10B), which has a high propensity to capture low energy "thermal" neutrons. The neutron cross section of 10B is 1,000 times more than that of other elements, such as nitrogen, hydrogen, or oxygen, that occur in tissue. In the second step, the patient is radiated with epithermal neutrons, the sources of which in the past have been nuclear reactors and now are accelerators that produce higher energy epithermal neutrons. After losing energy as they penetrate tissue, the resultant low energy "thermal" neutrons are captured by the 10B atoms. The resulting decay reaction yields high-energy alpha particles that kill the cancer cells that have taken up enough 10B.

The abscopal effect is a hypothesis in the treatment of metastatic cancer whereby shrinkage of untreated tumors occurs concurrently with shrinkage of tumors within the scope of the localized treatment. R.H. Mole proposed the term “abscopal” in 1953 to refer to effects of ionizing radiation “at a distance from the irradiated volume but within the same organism.”
Exposure to ionizing radiation is known to increase the future incidence of cancer, particularly leukemia. The mechanism by which this occurs is well understood, but quantitative models predicting the level of risk remain controversial. The most widely accepted model posits that the incidence of cancers due to ionizing radiation increases linearly with effective radiation dose at a rate of 5.5% per sievert; if correct, natural background radiation is the most hazardous source of radiation to general public health, followed by medical imaging as a close second. Additionally, the vast majority of non-invasive cancers are non-melanoma skin cancers caused by ultraviolet radiation. Non-ionizing radio frequency radiation from mobile phones, electric power transmission, and other similar sources have been investigated as a possible carcinogen by the WHO's International Agency for Research on Cancer, but to date, no evidence of this has been observed.
Micro-Culture Kinetic (MiCK) assay, developed by DiaTech Oncology, is a clinical pathology test that measures apoptosis induced in specific patient's cancer cells via chemotherapy. The assay is performed by DiaTech Oncology in its CLAI-certified, CAP-accredited laboratory. The MiCK assay provides oncologists with a clinically relevant drug-sensitivity profile of tumor cells within an individual cancer patient.
Travel outside the Earth's protective atmosphere, magnetosphere, and in free fall can harm human health, and understanding such harm is essential for successful crewed spaceflight. Potential effects on the central nervous system (CNS) are particularly important. A vigorous ground-based cellular and animal model research program will help quantify the risk to the CNS from space radiation exposure on future long distance space missions and promote the development of optimized countermeasures.
Prophylactic cranial irradiation (PCI) is a technique used to combat the occurrence of metastasis to the brain in highly aggressive cancers that commonly metastasize to brain, most notably small-cell lung cancer. Radiation therapy is commonly used to treat known tumor occurrence in the brain, either with highly precise stereotactic radiation or therapeutic cranial irradiation. By contrast, PCI is intended as preemptive treatment in patients with no known current intracranial tumor, but with high likelihood for harboring occult microscopic disease and eventual occurrence. For small-cell lung cancer with limited and select cases of extensive disease, PCI has shown to reduce recurrence of brain metastases and improve overall survival in complete remission.
Ionizing radiation can cause biological effects which are passed on to offspring through the epigenome. The effects of radiation on cells has been found to be dependent on the dosage of the radiation, the location of the cell in regards to tissue, and whether the cell is a somatic or germ line cell. Generally, ionizing radiation appears to reduce methylation of DNA in cells.
References
- ↑ Hoffman, Robert M. (1991). "In vitro sensitivity assays in cancer: A review, analysis, and prognosis". Journal of Clinical Laboratory Analysis. 5 (2): 133–43. doi:10.1002/jcla.1860050211. PMID 2023059.
- ↑ Ngunjiri, J. M.; Sekellick, M. J.; Marcus, P. I. (2008). "Clonogenic Assay of Type a Influenza Viruses Reveals Noninfectious Cell-Killing (Apoptosis-Inducing) Particles". Journal of Virology. 82 (6): 2673–80. doi:10.1128/JVI.02221-07. PMC 2258965 . PMID 18184709.
- ↑ Transcript of TWiV interview@http://www.twiv.tv/TWiV197-082612.pdf
- ↑ Franken, Nicolaas A P; Rodermond, Hans M; Stap, Jan; Haveman, Jaap; Van Bree, Chris (2006). "Clonogenic assay of cells in vitro". Nature Protocols. 1 (5): 2315–9. doi:10.1038/nprot.2006.339. PMID 17406473.
- ↑ Hamburger, Anne W. (1987). "The Human Tumor Clonogenic Assay as a Model System in Cell Biology". The International Journal of Cell Cloning. 5 (2): 89–107. doi:10.1002/stem.5530050202.
- ↑ Niyazi, Maximilian; Niyazi, Ismat; Belka, Claus (2007). "Counting colonies of clonogenic assays by using densitometric software". Radiation Oncology. 2: 4. doi:10.1186/1748-717X-2-4. PMC 1770926 . PMID 17212832.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ↑ Dahle, Jostein; Kakar, Manish; Steen, Harald B.; Kaalhus, Olav (2004). "Automated counting of mammalian cell colonies by means of a flat bed scanner and image processing". Cytometry. 60A (2): 182–8. doi:10.1002/cyto.a.20038.
- ↑ Carney, DN; Winkler, CF (1985). "In vitro assays of chemotherapeutic sensitivity". Important advances in oncology: 78–103. PMID 3916747.
- ↑ Liu, Q; Meng, W (2015). "Adapting a Drug Screening Platform to Discover Associations of Molecular Targeted Radiosensitizers with Genomic Biomarkers". Molecular Cancer Research. 13: 713–720. doi:10.1158/1541-7786.MCR-14-0570. PMC 4410013 . PMID 25667133.