
In chemistry, the dispersity is a measure of the heterogeneity of sizes of molecules or particles in a mixture. A collection of objects is called uniform if the objects have the same size, shape, or mass. A sample of objects that have an inconsistent size, shape and mass distribution is called non-uniform. The objects can be in any form of chemical dispersion, such as particles in a colloid, droplets in a cloud, crystals in a rock, or polymer macromolecules in a solution or a solid polymer mass. Polymers can be described by molecular mass distribution; a population of particles can be described by size, surface area, and/or mass distribution; and thin films can be described by film thickness distribution.

In industrial process engineering, mixing is a unit operation that involves manipulation of a heterogeneous physical system with the intent to make it more homogeneous. Familiar examples include pumping of the water in a swimming pool to homogenize the water temperature, and the stirring of pancake batter to eliminate lumps (deagglomeration).
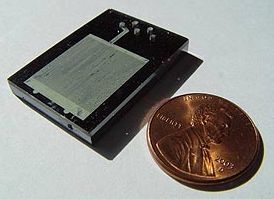
A microreactor or microstructured reactor or microchannel reactor is a device in which chemical reactions take place in a confinement with typical lateral dimensions below 1 mm; the most typical form of such confinement are microchannels. Microreactors are studied in the field of micro process engineering, together with other devices in which physical processes occur. The microreactor is usually a continuous flow reactor. Microreactors can offer many advantages over conventional scale reactors, including improvements in energy efficiency, reaction speed and yield, safety, reliability, scalability, on-site/on-demand production, and a much finer degree of process control.

A chemical reactor is an enclosed volume in which a chemical reaction takes place. In chemical engineering, it is generally understood to be a process vessel used to carry out a chemical reaction, which is one of the classic unit operations in chemical process analysis. The design of a chemical reactor deals with multiple aspects of chemical engineering. Chemical engineers design reactors to maximize net present value for the given reaction. Designers ensure that the reaction proceeds with the highest efficiency towards the desired output product, producing the highest yield of product while requiring the least amount of money to purchase and operate. Normal operating expenses include energy input, energy removal, raw material costs, labor, etc. Energy changes can come in the form of heating or cooling, pumping to increase pressure, frictional pressure loss or agitation.
Micro process engineering is the science of conducting chemical or physical processes inside small volumina, typically inside channels with diameters of less than 1 mm (microchannels) or other structures with sub-millimeter dimensions. These processes are usually carried out in continuous flow mode, as opposed to batch production, allowing a throughput high enough to make micro process engineering a tool for chemical production. Micro process engineering is therefore not to be confused with microchemistry, which deals with very small overall quantities of matter.

A chemical plant is an industrial process plant that manufactures chemicals, usually on a large scale. The general objective of a chemical plant is to create new material wealth via the chemical or biological transformation and or separation of materials. Chemical plants use specialized equipment, units, and technology in the manufacturing process. Other kinds of plants, such as polymer, pharmaceutical, food, and some beverage production facilities, power plants, oil refineries or other refineries, natural gas processing and biochemical plants, water and wastewater treatment, and pollution control equipment use many technologies that have similarities to chemical plant technology such as fluid systems and chemical reactor systems. Some would consider an oil refinery or a pharmaceutical or polymer manufacturer to be effectively a chemical plant.
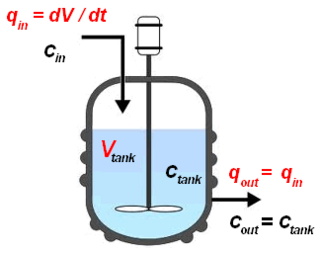
The continuous stirred-tank reactor (CSTR), also known as vat- or backmix reactor, mixed flow reactor (MFR), or a continuous-flow stirred-tank reactor (CFSTR), is a common model for a chemical reactor in chemical engineering and environmental engineering. A CSTR often refers to a model used to estimate the key unit operation variables when using a continuous agitated-tank reactor to reach a specified output. The mathematical model works for all fluids: liquids, gases, and slurries.
In physics, a mass balance, also called a material balance, is an application of conservation of mass to the analysis of physical systems. By accounting for material entering and leaving a system, mass flows can be identified which might have been unknown, or difficult to measure without this technique. The exact conservation law used in the analysis of the system depends on the context of the problem, but all revolve around mass conservation, i.e., that matter cannot disappear or be created spontaneously.

A static mixer is a device for the continuous mixing of fluid materials, without moving components. Normally the fluids to be mixed are liquid, but static mixers can also be used to mix gas streams, disperse gas into liquid or blend immiscible liquids. The energy needed for mixing comes from a loss in pressure as fluids flow through the static mixer. One design of static mixer is the plate-type mixer and another common device type consists of mixer elements contained in a cylindrical (tube) or squared housing. Mixer size can vary from about 6 mm to 6 meters diameter. Typical construction materials for static mixer components include stainless steel, polypropylene, Teflon, PVDF, PVC, CPVC and polyacetal. The latest designs involve static mixing elements made of glass-lined steel.

The plug flow reactor model is a model used to describe chemical reactions in continuous, flowing systems of cylindrical geometry. The PFR model is used to predict the behavior of chemical reactors of such design, so that key reactor variables, such as the dimensions of the reactor, can be estimated.
A batch reactor is a chemical reactor in which a non-continuous reaction is conducted, i.e., one where the reactants, products and solvent do not flow in or out of the vessel during the reaction until the target reaction conversion is achieved. By extension, the expression is somehow inappropriately used for other batch fluid processing operations that do not involve a chemical reaction, such as solids dissolution, product mixing, batch distillation, crystallization, and liquid/liquid extraction. In such cases, however, they may not be referred to as reactors but rather with a term specific to the function they perform.
In flow chemistry, also called reactor engineering, a chemical reaction is run in a continuously flowing stream rather than in batch production. In other words, pumps move fluid into a reactor, and where tubes join one another, the fluids contact one another. If these fluids are reactive, a reaction takes place. Flow chemistry is a well-established technique for use at a large scale when manufacturing large quantities of a given material. However, the term has only been coined recently for its application on a laboratory scale by chemists and describes small pilot plants, and lab-scale continuous plants. Often, microreactors are used.

A reaction calorimeter is a calorimeter that measures the amount of energy released (exothermic) or absorbed (endothermic) by a chemical reaction.

A fluidized bed reactor (FBR) is a type of reactor device that can be used to carry out a variety of multiphase chemical reactions. In this type of reactor, a fluid is passed through a solid granular material at high enough speeds to suspend the solid and cause it to behave as though it were a fluid. This process, known as fluidization, imparts many important advantages to an FBR. As a result, FBRs are used for many industrial applications.
For both chemical and biological engineering, Semibatch (semiflow) reactors operate much like batch reactors in that they take place in a single stirred tank with similar equipment. However, they are modified to allow reactant addition and/or product removal in time.
In fluid thermodynamics, a heat transfer fluid is a gas or liquid that takes part in heat transfer by serving as an intermediary in cooling on one side of a process, transporting and storing thermal energy, and heating on another side of a process. Heat transfer fluids are used in countless applications and industrial processes requiring heating or cooling, typically in a closed circuit and in continuous cycles. Cooling water, for instance, cools an engine, while heating water in a hydronic heating system heats the radiator in a room.
The circulating fluidized bed (CFB) is a type of Fluidized bed combustion that utilizes a recirculating loop for even greater efficiency of combustion. while achieving lower emission of pollutants. Reports suggest that up to 95% of pollutants can be absorbed before being emitted into the atmosphere. The technology is limited in scale however, due to its extensive use of limestone, and the fact that it produces waste byproducts.
Ebullated bed reactors are a type of fluidized bed reactor that utilizes ebullition, or bubbling, to achieve appropriate distribution of reactants and catalysts. The ebullated-bed technology utilizes a three-phase reactor, and is most applicable for exothermic reactions and for feedstocks which are difficult to process in fixed-bed or plug flow reactors due to high levels of contaminants. Ebullated bed reactors offer high-quality, continuous mixing of liquid and catalyst particles. The advantages of a good back-mixed bed include excellent temperature control and, by reducing bed plugging and channeling, low and constant pressure drops. Therefore, ebullated bed reactors have the characteristics of stirred reactor type operation with a fluidized catalyst.
The residence time of a fluid parcel is the total time that the parcel has spent inside a control volume (e.g.: a chemical reactor, a lake, a human body). The residence time of a set of parcels is quantified in terms of the frequency distribution of the residence time in the set, which is known as residence time distribution (RTD), or in terms of its average, known as mean residence time.

A Continuous Oscillatory Baffled Reactor (COBR) is a specially designed chemical reactor to achieve plug flow under laminar flow conditions. Achieving plug flow has previously been limited to either a large number of continuous stir tank reactors (CSTR) in series or conditions with high turbulent flow. The technology incorporates annular baffles to a tubular reactor framework to create eddies when liquid is pushed up through the tube. Likewise, when liquid is on a downstroke through the tube, eddies are created on the other side of the baffles. Eddy generation on both sides of the baffles creates very effective mixing while still maintaining plug flow. By using COBR, potentially higher yields of product can be made with greater control and reduced waste.

















