
Bacillus thuringiensis is a gram-positive, soil-dwelling bacterium, the most commonly used biological pesticide worldwide. B. thuringiensis also occurs naturally in the gut of caterpillars of various types of moths and butterflies, as well on leaf surfaces, aquatic environments, animal feces, insect-rich environments, and flour mills and grain-storage facilities. It has also been observed to parasitize other moths such as Cadra calidella—in laboratory experiments working with C. calidella, many of the moths were diseased due to this parasite.

A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents.

Bacillus thuringiensis serotype israelensis (Bti) is a group of bacteria used as biological control agents for larvae stages of certain dipterans. Bti produces toxins which are effective in killing various species of mosquitoes, fungus gnats, and blackflies, while having almost no effect on other organisms. The major advantage of B. thuringiensis products is that they are thought to affect few non-target species. However, even though Bti may have minimal direct effects on non-target organisms, it may potentially be associated with knock-on effects on food webs and other ecosystem properties, including biodiversity and ecosystem functioning.

An enterotoxin is a protein exotoxin released by a microorganism that targets the intestines. They can be chromosomally or plasmid encoded. They are heat labile (>60⁰), of low molecular weight and water-soluble. Enterotoxins are frequently cytotoxic and kill cells by altering the apical membrane permeability of the mucosal (epithelial) cells of the intestinal wall. They are mostly pore-forming toxins, secreted by bacteria, that assemble to form pores in cell membranes. This causes the cells to die.
A latrotoxin is a high-molecular mass neurotoxin found in the venom of spiders of the genus Latrodectus as well as at least one species of another genus in the same family, Steatoda nobilis. Latrotoxins are the main active components of the venom and are responsible for the symptoms of latrodectism.

Diphtheria toxin is an exotoxin secreted mainly by Corynebacterium diphtheriae but also by Corynebacterium ulcerans and Corynebacterium pseudotuberculosis. the pathogenic bacterium that causes diphtheria. The toxin gene is encoded by a prophage called corynephage β. The toxin causes the disease in humans by gaining entry into the cell cytoplasm and inhibiting protein synthesis.
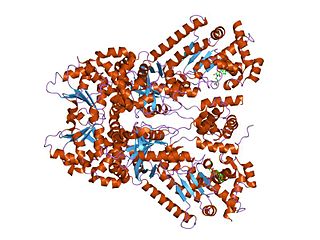
Anthrax toxin is a three-protein exotoxin secreted by virulent strains of the bacterium, Bacillus anthracis—the causative agent of anthrax. The toxin was first discovered by Harry Smith in 1954. Anthrax toxin is composed of a cell-binding protein, known as protective antigen (PA), and two enzyme components, called edema factor (EF) and lethal factor (LF). These three protein components act together to impart their physiological effects. Assembled complexes containing the toxin components are endocytosed. In the endosome, the enzymatic components of the toxin translocate into the cytoplasm of a target cell. Once in the cytosol, the enzymatic components of the toxin disrupts various immune cell functions, namely cellular signaling and cell migration. The toxin may even induce cell lysis, as is observed for macrophage cells. Anthrax toxin allows the bacteria to evade the immune system, proliferate, and ultimately kill the host animal. Research on anthrax toxin also provides insight into the generation of macromolecular assemblies, and on protein translocation, pore formation, endocytosis, and other biochemical processes.

Pore-forming proteins are usually produced by bacteria, and include a number of protein exotoxins but may also be produced by other organisms such as apple snails that produce perivitellin-2 or earthworms, who produce lysenin. They are frequently cytotoxic, as they create unregulated pores in the membrane of targeted cells.

Hemolysins or haemolysins are lipids and proteins that cause lysis of red blood cells by disrupting the cell membrane. Although the lytic activity of some microbe-derived hemolysins on red blood cells may be of great importance for nutrient acquisition, many hemolysins produced by pathogens do not cause significant destruction of red blood cells during infection. However, hemolysins are often capable of lysing red blood cells in vitro.
The Membrane Attack Complex/Perforin (MACPF) superfamily, sometimes referred to as the MACPF/CDC superfamily, is named after a domain that is common to the membrane attack complex (MAC) proteins of the complement system and perforin (PF). Members of this protein family are pore-forming toxins (PFTs). In eukaryotes, MACPF proteins play a role in immunity and development.

Alpha-toxin, also known as alpha-hemolysin (Hla), is the major cytotoxic agent released by bacterium Staphylococcus aureus and the first identified member of the pore forming beta-barrel toxin family. This toxin consists mostly of beta-sheets (68%) with only about 10% alpha-helices. The hly gene on the S. aureus chromosome encodes the 293 residue protein monomer, which forms heptameric units on the cellular membrane to form a complete beta-barrel pore. This structure allows the toxin to perform its major function, development of pores in the cellular membrane, eventually causing cell death.
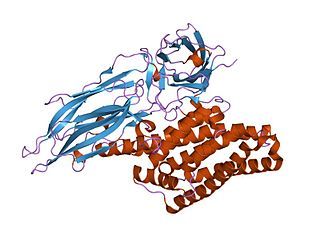
Delta endotoxins (δ-endotoxins) are pore-forming toxins produced by Bacillus thuringiensis species of bacteria. They are useful for their insecticidal action and are the primary toxin produced by Bt maize/corn. During spore formation the bacteria produce crystals of such proteins that are also known as parasporal bodies, next to the endospores; as a result some members are known as a parasporin. The Cyt (cytolytic) toxin group is a group of delta-endotoxins different from the Cry group.
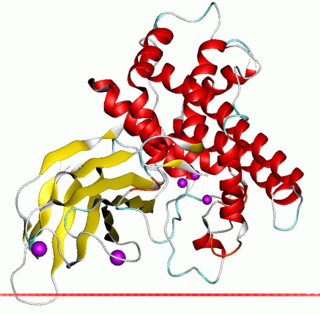
In molecular biology, zinc-dependent phospholipases C is a family of bacterial phospholipases C enzymes, some of which are also known as alpha toxins.

Clostridium septicum is a gram positive, spore forming, obligate anaerobic bacterium.
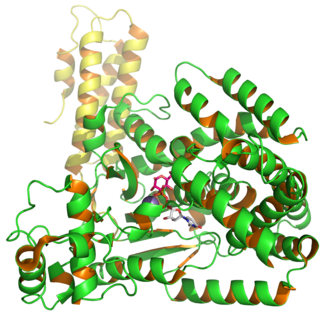
Clostridium difficile toxin B is a cytotoxin produced by the bacteria Clostridioides difficile, formerly known as Clostridium difficile. It is one of two major kinds of toxins produced by C. difficile, the other being an related enterotoxin. Both are very potent and lethal.

María Alejandra Bravo de la Parra is a Mexican biochemist who was laureated with the 2010 L'Oréal-UNESCO Award for Women in Science – Latin America for her work on a bacterial toxin that acts as a powerful insecticide. Bravo has co-authored multiple papers with her husband Mario Soberon.
The RTX toxin superfamily is a group of cytolysins and cytotoxins produced by bacteria. There are over 1000 known members with a variety of functions. The RTX family is defined by two common features: characteristic repeats in the toxin protein sequences, and extracellular secretion by the type I secretion systems (T1SS). The name RTX refers to the glycine and aspartate-rich repeats located at the C-terminus of the toxin proteins, which facilitate export by a dedicated T1SS encoded within the rtx operon.
Clostridium perfringens beta toxin is one of the four major lethal protein toxins produced by Clostridium perfringens Type B and Type C strains. It is a necrotizing agent and it induces hypertension by release of catecholamine. It has been shown to cause necrotic enteritis in mammals and induces necrotizing intestinal lesions in the rabbit ileal loop model. C. perfringens beta toxin is susceptible to breakdown by proteolytic enzymes, particularly trypsin. Beta toxin is therefore highly lethal to infant mammals because of trypsin inhibitors present in the colostrum.
The thiol-activated Cholesterol-dependent Cytolysin(CDC) family is a member of the MACPF superfamily. Cholesterol dependent cytolysins are a family of β-barrel pore-forming exotoxins that are secreted by gram-positive bacteria. CDCs are secreted as water-soluble monomers of 50-70 kDa, that when bound to the target cell, form a circular homo-oligomeric complex containing as many as 40 monomers. Through multiple conformational changes, the β-barrel transmembrane structure is formed and inserted into the target cell membrane. The presence of cholesterol in the target membrane is required for pore formation, though the presence of cholesterol is not required by all CDCs for binding. For example, intermedilysin secreted by Streptococcus intermedius will bind only to target membranes containing a specific protein receptor, independent of the presence of cholesterol, but cholesterol is required by intermedilysin for pore formation. While the lipid environment of cholesterol in the membrane can affect toxin binding, the exact molecular mechanism that cholesterol regulates the cytolytic activity of the CDC is not fully understood.
Cry34Ab1 is one member of a binary Bacillus thuringiensis (Bt) crystal protein set isolated from Bt strain PS149B1. The protein exists as a 14 kDa aegerolysin that, in presence of Cry35Ab1, exhibits insecticidal activity towards Western Corn Rootworm. The protein has been transformed into maize plants under the commercialized events 4114 (DP-ØØ4114-3) by Pioneer Hi-Bred and 59122 (DAS-59122-7) by Dow AgroSciences. These events have, in turn, been bred into multiple trait stacks in additional products.












