
A ceramic is any of the various hard, brittle, heat-resistant, and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain, and brick.

Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth.

A gemstone is a piece of mineral crystal which, when cut or polished, is used to make jewelry or other adornments. Certain rocks and occasionally organic materials that are not minerals may also be used for jewelry and are therefore often considered to be gemstones as well. Most gemstones are hard, but some softer minerals such as brazilianite may be used in jewelry because of their color or luster or other physical properties that have aesthetic value. However, generally speaking, soft minerals are not typically used as gemstones by virtue of their brittleness and lack of durability.

Zirconium is a chemical element; it has symbol Zr and atomic number 40. The name zirconium is derived from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian zargun. It is a lustrous, grey-white, strong transition metal that closely resembles hafnium and, to a lesser extent, titanium.

Rutile is an oxide mineral composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite.

The skull crucible process was developed at the Lebedev Physical Institute in Moscow to manufacture cubic zirconia. It was invented to solve the problem of cubic zirconia's melting-point being too high for even platinum crucibles.

Zirconium dioxide, sometimes known as zirconia, is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabilized cubic structured zirconia, cubic zirconia, is synthesized in various colours for use as a gemstone and a diamond simulant.

Moissanite is naturally occurring silicon carbide and its various crystalline polymorphs. It has the chemical formula SiC and is a rare mineral, discovered by the French chemist Henri Moissan in 1893. Silicon carbide or moissanite is useful for commercial and industrial applications due to its hardness, optical properties and thermal conductivity.
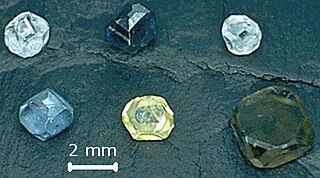
Lab-grown diamond is diamond that is produced in a controlled technological process. Unlike diamond simulants, synthetic diamonds are composed of the same material as naturally formed diamonds—pure carbon crystallized in an isotropic 3D form—and share identical chemical and physical properties.

Strontium titanate is an oxide of strontium and titanium with the chemical formula SrTiO3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure. At low temperatures it approaches a ferroelectric phase transition with a very large dielectric constant ~104 but remains paraelectric down to the lowest temperatures measured as a result of quantum fluctuations, making it a quantum paraelectric. It was long thought to be a wholly artificial material, until 1982 when its natural counterpart—discovered in Siberia and named tausonite—was recognised by the IMA. Tausonite remains an extremely rare mineral in nature, occurring as very tiny crystals. Its most important application has been in its synthesized form wherein it is occasionally encountered as a diamond simulant, in precision optics, in varistors, and in advanced ceramics.

Diamond is the allotrope of carbon in which the carbon atoms are arranged in the specific type of cubic lattice called diamond cubic. It is a crystal that is transparent to opaque and which is generally isotropic. Diamond is the hardest naturally occurring material known. Yet, due to important structural brittleness, bulk diamond's toughness is only fair to good. The precise tensile strength of bulk diamond is little known; however, compressive strength up to 60 GPa has been observed, and it could be as high as 90–100 GPa in the form of micro/nanometer-sized wires or needles, with a corresponding maximum tensile elastic strain in excess of 9%. The anisotropy of diamond hardness is carefully considered during diamond cutting. Diamond has a high refractive index (2.417) and moderate dispersion (0.044) properties that give cut diamonds their brilliance. Scientists classify diamonds into four main types according to the nature of crystallographic defects present. Trace impurities substitutionally replacing carbon atoms in a diamond's crystal structure, and in some cases structural defects, are responsible for the wide range of colors seen in diamond. Most diamonds are electrical insulators and extremely efficient thermal conductors. Unlike many other minerals, the specific gravity of diamond crystals (3.52) has rather small variation from diamond to diamond.

A diamond simulant, diamond imitation or imitation diamond is an object or material with gemological characteristics similar to those of a diamond. Simulants are distinct from synthetic diamonds, which are actual diamonds exhibiting the same material properties as natural diamonds. Enhanced diamonds are also excluded from this definition. A diamond simulant may be artificial, natural, or in some cases a combination thereof. While their material properties depart markedly from those of diamond, simulants have certain desired characteristics—such as dispersion and hardness—which lend themselves to imitation. Trained gemologists with appropriate equipment are able to distinguish natural and synthetic diamonds from all diamond simulants, primarily by visual inspection.

Yttrium aluminium garnet (YAG, Y3Al5O12) is a synthetic crystalline material of the garnet group. It is a cubic yttrium aluminium oxide phase, with other examples being YAlO3 (YAP) in a hexagonal or an orthorhombic, perovskite-like form, and the monoclinic Y4Al2O9 (YAM).

Yttrium oxide, also known as yttria, is Y2O3. It is an air-stable, white solid substance.

Zirconium carbide (ZrC) is an extremely hard refractory ceramic material, commercially used in tool bits for cutting tools. It is usually processed by sintering.

A ceramic knife is a knife with a ceramic blade typically made from zirconium dioxide (ZrO2; also known as zirconia), rather than the steel used for most knives. Ceramic knife blades are usually produced through the dry-pressing and firing of powdered zirconia using solid-state sintering. The blades typically score 8.5 on the Mohs scale of mineral hardness, compared to 4.5 for normal steel and 7.5 to 8 for hardened steel and 10 for diamond. The resultant blade has a hard edge that stays sharp for much longer than conventional steel blades. However, the blade is brittle, subject to chipping, and will break rather than flex if twisted. The ceramic blade is sharpened by grinding the edges with a diamond-dust-coated grinding wheel.

Yttria-stabilized zirconia (YSZ) is a ceramic in which the cubic crystal structure of zirconium dioxide is made stable at room temperature by an addition of yttrium oxide. These oxides are commonly called "zirconia" (ZrO2) and "yttria" (Y2O3), hence the name.
Gadolinium Gallium Garnet is a synthetic crystalline material of the garnet group, with good mechanical, thermal, and optical properties. It is typically colorless. It has a cubic lattice, a density of 7.08 g/cm3 and its Mohs hardness is variously noted as 6.5 and 7.5. Its crystals are produced with the Czochralski method. During production, various dopants can be added for colour modification. The material is also used in fabrication of various optical components and as a substrate material for magneto–optical films. It also finds use in jewelry as a diamond simulant. GGG can also be used as a seed substrate for the growth of other garnets such as yttrium iron garnet.

The Shelby Gem Factory was the production facility of ICT Incorporated, a company in Shelby, Michigan, United States, that manufactured artificial gemstones through proprietary processes. ICT began operations in 1970 and closed in December 2019.
Ceria-zirconia is a solid solution of cerium(IV) oxide (CeO2, also known as ceria) and zirconium oxide (ZrO2, also known as zirconia).

































