
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.

Cis–trans isomerism, also known as geometric isomerism or configurational isomerism, is a term used in chemistry that concerns the spatial arrangement of atoms within molecules. The prefixes "cis" and "trans" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, cis indicates that the functional groups (substituents) are on the same side of some plane, while trans conveys that they are on opposing (transverse) sides. Cis–trans isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. Cis-trans notation does not always correspond to E–Z isomerism, which is an absolute stereochemical description. In general, cis–trans stereoisomers contain double bonds that do not rotate, or they may contain ring structures, where the rotation of bonds is restricted or prevented. Cis and trans isomers occur both in organic molecules and in inorganic coordination complexes. Cis and trans descriptors are not used for cases of conformational isomerism where the two geometric forms easily interconvert, such as most open-chain single-bonded structures; instead, the terms "syn" and "anti" are used.

In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 of one alpha-amino acid and N2 of another, along a peptide or protein chain.

In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group -NH
2 but is rather a secondary amine. The secondary amine nitrogen is in the protonated NH2+ form under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG).
Isomerases are a general class of enzymes that convert a molecule from one isomer to another. Isomerases facilitate intramolecular rearrangements in which bonds are broken and formed. The general form of such a reaction is as follows:

A meso compound or meso isomer is a non-optically active member of a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereogenic centers, the molecule is not chiral. A meso compound is "superposable" on its mirror image. Two objects can be superposed if all aspects of the objects coincide and it does not produce a "(+)" or "(-)" reading when analyzed with a polarimeter. The name is derived from the Greek mésos meaning “middle”.
A cycloalkene or cycloolefin is a type of alkene hydrocarbon which contains a closed ring of carbon atoms and either one or more double bonds, but has no aromatic character. Some cycloalkenes, such as cyclobutene and cyclopentene, can be used as monomers to produce polymer chains. Due to geometrical considerations, smaller cycloalkenes are almost always the cis isomers, and the term cis tends to be omitted from the names. trans-Cycloalkenes with 7 or fewer carbons in the ring will not occur under normal conditions because of the large amount of ring strain needed. In larger rings, cis–trans isomerism of the double bond may occur. This stability pattern forms part of the origin of Bredt's rule, the observation that alkenes do not form at the bridgehead of many types of bridged ring systems because the alkene would necessarily be trans in one of the rings.
In organic chemistry, cyclohexane conformations are any of several three-dimensional shapes adopted by molecules of cyclohexane. Because many compounds feature structurally similar six-membered rings, the structure and dynamics of cyclohexane are important prototypes of a wide range of compounds.

In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds. While any two arrangements of atoms in a molecule that differ by rotation about single bonds can be referred to as different conformations, conformations that correspond to local minima on the potential energy surface are specifically called conformational isomers or conformers. Conformations that correspond to local maxima on the energy surface are the transition states between the local-minimum conformational isomers. Rotations about single bonds involve overcoming a rotational energy barrier to interconvert one conformer to another. If the energy barrier is low, there is free rotation and a sample of the compound exists as a rapidly equilibrating mixture of multiple conformers; if the energy barrier is high enough then there is restricted rotation, a molecule may exist for a relatively long time period as a stable rotational isomer or rotamer. When the time scale for interconversion is long enough for isolation of individual rotamers, the isomers are termed atropisomers. The ring-flip of substituted cyclohexanes constitutes another common form of conformational isomerism.
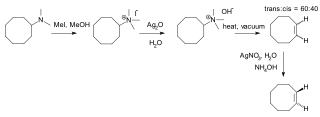
Hofmann elimination is an elimination reaction of an amine to form alkenes. The least stable alkene, called the Hofmann product, is formed. This tendency, known as the Hofmann alkene synthesis rule, is in contrast to usual elimination reactions, where Zaitsev's rule predicts the formation of the most stable alkene. It is named after its discoverer, August Wilhelm von Hofmann.

Decalin, a bicyclic organic compound, is an industrial solvent. A colorless liquid with an aromatic odor, it is used as a solvent for many resins or fuel additives. It is the saturated analog of naphthalene and can be prepared from it by hydrogenation in the presence of a catalyst. Decalin easily forms explosive organic peroxides upon storage in the presence of air.
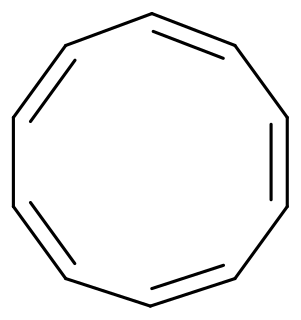
Cyclodecapentaene or [10]annulene is an annulene with molecular formula C10H10. This organic compound is a conjugated 10 pi electron cyclic system and according to Huckel's rule it should display aromaticity. It is not aromatic, however, because various types of ring strain destabilize an all-planar geometry. The all-cis isomer (1), a fully convex decagon, would have bond angles of 144°, which creates large amounts of angle strain relative to the ideal 120° for sp2 atomic hybridization. Instead, the all-cis isomer can adopt a planar boat-like conformation (2) to relieve the angle strain. This is still unstable because of the relative higher strain in boat shaped compared to the next planar trans, cis, trans, cis, cis isomer (3). Yet even this isomer is also unstable, suffering from steric repulsion between the two internal hydrogen atoms. The nonplanar trans, cis, cis, cis, cis isomer (4) is the most stable of all the possible isomers.

In organic chemistry, transannular strain is the unfavorable interactions of ring substituents on non-adjacent carbons. These interactions, called transannular interactions, arise from a lack of space in the interior of the ring, which forces substituents into conflict with one another. In medium-sized cycloalkanes, which have between 8 and 11 carbons constituting the ring, transannular strain can be a major source of the overall strain, especially in some conformations, to which there is also contribution from large-angle strain and Pitzer strain. In larger rings, transannular strain drops off until the ring is sufficiently large that it can adopt conformations devoid of any negative interactions.
Cycloheptene is a 7-membered cycloalkene with a flash point of −6.7 °C. It is a raw material in organic chemistry and a monomer in polymer synthesis. Cycloheptene can exist as either the cis- or the trans-isomer.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.
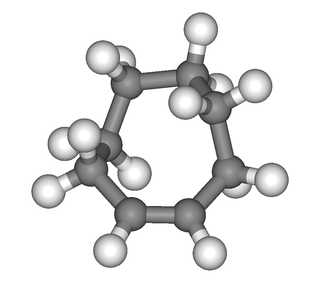
trans-Cyclooctene is a cyclic hydrocarbon with the formula [–(CH2)6CH=CH–], where the two C–C single bonds adjacent to the double bond are on opposite sides of the latter's plane. It is a colorless liquid with a disagreeable odor.

cis-Cyclooctene is a cycloalkene with the formula (CH2)6(CH)2. It is a colorless liquid that is used industrially to produce a polymer. It is also a ligand in organometallic chemistry.

In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.

Methylcyclohexene refers to any one of three organic compounds consisting of cyclohexene with a methyl group substituent. The location of the methyl group relative to the cyclohexene double bond creates the three different structural isomers. These compounds are generally used as a reagent or intermediate to derive other organic compounds.















