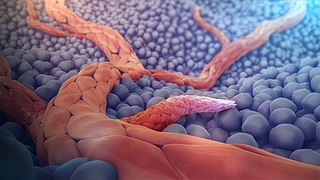
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, formed in the earlier stage of vasculogenesis. Angiogenesis continues the growth of the vasculature by processes of sprouting and splitting. Vasculogenesis is the embryonic formation of endothelial cells from mesoderm cell precursors, and from neovascularization, although discussions are not always precise. The first vessels in the developing embryo form through vasculogenesis, after which angiogenesis is responsible for most, if not all, blood vessel growth during development and in disease.

Itraconazole, sometimes abbreviated ITZ, is an antifungal medication used to treat a number of fungal infections. This includes aspergillosis, blastomycosis, coccidioidomycosis, histoplasmosis, and paracoccidioidomycosis. It may be given by mouth or intravenously.
An angiogenesis inhibitor is a substance that inhibits the growth of new blood vessels (angiogenesis). Some angiogenesis inhibitors are endogenous and a normal part of the body's control and others are obtained exogenously through pharmaceutical drugs or diet.

Endostatin is a naturally occurring, 20-kDa C-terminal fragment derived from type XVIII collagen. It is reported to serve as an anti-angiogenic agent, similar to angiostatin and thrombospondin.
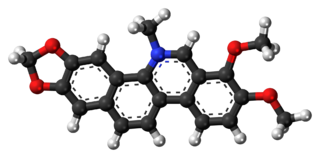
Chelerythrine is a benzophenanthridine alkaloid present in the plant Chelidonium majus. It is a potent, selective, and cell-permeable protein kinase C inhibitor in vitro. And an efficacious antagonist of G-protein-coupled CB1 receptors. This molecule also exhibits anticancer qualities and it has served as a base for many potential novel drugs against cancer. Structurally, this molecule has two distinct conformations, one being a positively charged iminium form, and the other being an uncharged form, a pseudo-base.
Cytochalasins are fungal metabolites that have the ability to bind to actin filaments and block polymerization and the elongation of actin. As a result of the inhibition of actin polymerization, cytochalasins can change cellular morphology, inhibit cellular processes such as cell division, and even cause cells to undergo apoptosis. Cytochalasins have the ability to permeate cell membranes, prevent cellular translocation and cause cells to enucleate. Cytochalasins can also have an effect on other aspects of biological processes unrelated to actin polymerization. For example, cytochalasin A and cytochalasin B can also inhibit the transport of monosaccharides across the cell membrane, cytochalasin H has been found to regulate plant growth, cytochalasin D inhibits protein synthesis and cytochalasin E prevents angiogenesis.
The epoxyeicosatrienoic acids or EETs are signaling molecules formed within various types of cells by the metabolism of arachidonic acid by a specific subset of Cytochrome P450 enzymes termed cytochrome P450 epoxygenases. These nonclassic eicosanoids are generally short-lived, being rapidly converted from epoxides to less active or inactive dihydroxy-eicosatrienoic acids (diHETrEs) by a widely distributed cellular enzyme, Soluble epoxide hydrolase (sEH), also termed Epoxide hydrolase 2. The EETs consequently function as transiently acting, short-range hormones; that is, they work locally to regulate the function of the cells that produce them or of nearby cells. The EETs have been most studied in animal models where they show the ability to lower blood pressure possibly by a) stimulating arterial vasorelaxation and b) inhibiting the kidney's retention of salts and water to decrease intravascular blood volume. In these models, EETs prevent arterial occlusive diseases such as heart attacks and brain strokes not only by their anti-hypertension action but possibly also by their anti-inflammatory effects on blood vessels, their inhibition of platelet activation and thereby blood clotting, and/or their promotion of pro-fibrinolytic removal of blood clots. With respect to their effects on the heart, the EETs are often termed cardio-protective. Beyond these cardiovascular actions that may prevent various cardiovascular diseases, studies have implicated the EETs in the pathological growth of certain types of cancer and in the physiological and possibly pathological perception of neuropathic pain. While studies to date imply that the EETs, EET-forming epoxygenases, and EET-inactivating sEH can be manipulated to control a wide range of human diseases, clinical studies have yet to prove this. Determination of the role of the EETS in human diseases is made particularly difficult because of the large number of EET-forming epoxygenases, large number of epoxygenase substrates other than arachidonic acid, and the large number of activities, some of which may be pathological or injurious, that the EETs possess.

Thrombospondin 1, abbreviated as THBS1, is a protein that in humans is encoded by the THBS1 gene.
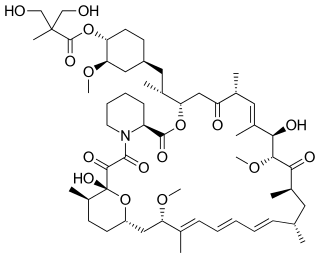
Temsirolimus, sold under the brand name Torisel, is an intravenous drug for the treatment of renal cell carcinoma (RCC), developed by Wyeth Pharmaceuticals and approved by the U.S. Food and Drug Administration (FDA) in May 2007, and was also approved by the European Medicines Agency (EMA) in November 2007. It is a derivative and prodrug of sirolimus.

Pigment epithelium-derived factor (PEDF) also known as serpin F1 (SERPINF1), is a multifunctional secreted protein that has anti-angiogenic, anti-tumorigenic, and neurotrophic functions. Found in vertebrates, this 50 kDa protein is being researched as a therapeutic candidate for treatment of such conditions as choroidal neovascularization, heart disease, and cancer. In humans, pigment epithelium-derived factor is encoded by the SERPINF1 gene.

PFKFB3 is a gene that encodes the 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 enzyme in humans. It is one of 4 tissue-specific PFKFB isoenzymes identified currently (PFKFB1-4).
A cartilage-derived angiogenesis inhibitor is an angiogenesis inhibitor produced from cartilage. Examples include the peptide troponin I and chondromodulin I. The antiangiogenic effect may be an inhibition of basement membrane degradation.
Semicarbazide-cadmium therapy was an experimental cancer therapy that was tested in several clinical trials in the Soviet Union during the 1960s. Semicarbazide is an irreversible inhibitor of semicarbazide-sensitive amine oxidase (SSAO), an enzyme possibly involved in exacerbation of inflammation. Cadmium is a heavy metal and can also induce apoptosis.
Safingol is a lyso-sphingolipid protein kinase inhibitor. It has the molecular formula C18H39NO2 and is a colorless solid. Medicinally, safingol has demonstrated promising anticancer potential as a modulator of multi-drug resistance and as an inducer of necrosis. The administration of safingol alone has not been shown to exert a significant effect on tumor cell growth. However, preclinical and clinical studies have shown that combining safingol with conventional chemotherapy agents such as fenretinide, vinblastine, irinotecan and mitomycin C can dramatically potentiate their antitumor effects. Currently in Phase I clinical trials, it is believed to be safe to co-administer with cisplatin.
Tumstatin is a protein fragment cleaved from collagen that serves as both an antiangiogenic and proapoptotic agent. It has similar function to canstatin, endostatin, restin, and arresten, which also affect angiogenesis. Angiogenesis is the growth of new blood vessels from pre-existing blood vessels, and is important in tumor growth and metastasis. Angiogenesis is stimulated by many growth factors, the most prevalent of which is vascular endothelial growth factor (VEGF).

Withaferin A is a steroidal lactone, derived from Acnistus arborescens, Withania somnifera and other members of family Solanaceae. It has been traditionally used in ayurvedic medicine. It is the first member of the withanolide class of ergostane type product to be discovered. This natural product has wide range of pharmacological activities including cardioprotective, anti-inflammatory, immuno-modulatory, anti-angiogenesis, anti-metastasis and anti-carcinogenic properties.

mTOR inhibitors are a class of drugs that inhibit the mechanistic target of rapamycin (mTOR), which is a serine/threonine-specific protein kinase that belongs to the family of phosphatidylinositol-3 kinase (PI3K) related kinases (PIKKs). mTOR regulates cellular metabolism, growth, and proliferation by forming and signaling through two protein complexes, mTORC1 and mTORC2. The most established mTOR inhibitors are so-called rapalogs, which have shown tumor responses in clinical trials against various tumor types.

Immunomodulatory imide drugs (IMiDs) are a class of immunomodulatory drugs containing an imide group. The IMiD class includes thalidomide and its analogues. These drugs may also be referred to as 'Cereblon modulators'. Cereblon (CRBN) is the protein targeted by this class of drugs.
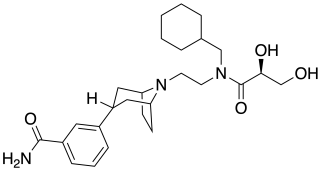
Axelopran is a drug which is under development by Theravance Biopharma and licensed to Glycyx for all indications. It acts as a peripherally acting μ-opioid receptor antagonist and also acts on κ-, and δ-opioid receptors, with similar affinity for the μ- and κ-opioid receptors and about an order of magnitude lower affinity for the δ-opioid receptor. Recent data suggests that μ-opioid antagonists have a direct effect on overall survival in patients with advanced cancer.
VEGFR-2 inhibitor, also known as kinase insert domain receptor(KDR) inhibitor, are tyrosine kinase receptor inhibitors that reduce angiogenesis or lymphangiogenesis, leading to anticancer activity. Generally they are small, synthesised molecules that bind competitively to the ATP-site of the tyrosine kinase domain. VEGFR-2 selective inhibitor can interrupt multiple signaling pathways involved in tumor, including proliferation, metastasis and angiogenesis.














