Related Research Articles
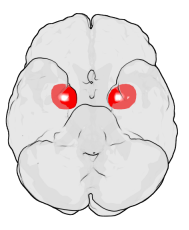
The amygdala is one of two almond-shaped clusters of nuclei located deep and medially within the temporal lobes of the brain's cerebrum in complex vertebrates, including humans. Shown to perform a primary role in the processing of memory, decision making, and emotional responses, the amygdalae are considered part of the limbic system. The term "amygdala" was first introduced by Karl Friedrich Burdach in 1822.

The limbic system, also known as the paleomammalian cortex, is a set of brain structures located on both sides of the thalamus, immediately beneath the medial temporal lobe of the cerebrum primarily in the forebrain.
The mesolimbic pathway, sometimes referred to as the reward pathway, is a dopaminergic pathway in the brain. The pathway connects the ventral tegmental area in the midbrain to the ventral striatum of the basal ganglia in the forebrain. The ventral striatum includes the nucleus accumbens and the olfactory tubercle.

The olfactory bulb is a neural structure of the vertebrate forebrain involved in olfaction, the sense of smell. It sends olfactory information to be further processed in the amygdala, the orbitofrontal cortex (OFC) and the hippocampus where it plays a role in emotion, memory and learning. The bulb is divided into two distinct structures: the main olfactory bulb and the accessory olfactory bulb. The main olfactory bulb connects to the amygdala via the piriform cortex of the primary olfactory cortex and directly projects from the main olfactory bulb to specific amygdala areas. The accessory olfactory bulb resides on the dorsal-posterior region of the main olfactory bulb and forms a parallel pathway. Destruction of the olfactory bulb results in ipsilateral anosmia, while irritative lesions of the uncus can result in olfactory and gustatory hallucinations.

Pavlovian fear conditioning is a behavioral paradigm in which organisms learn to predict aversive events. It is a form of learning in which an aversive stimulus is associated with a particular neutral context or neutral stimulus, resulting in the expression of fear responses to the originally neutral stimulus or context. This can be done by pairing the neutral stimulus with an aversive stimulus. Eventually, the neutral stimulus alone can elicit the state of fear. In the vocabulary of classical conditioning, the neutral stimulus or context is the "conditional stimulus" (CS), the aversive stimulus is the "unconditional stimulus" (US), and the fear is the "conditional response" (CR).
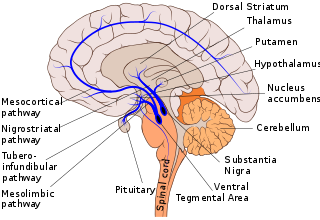
Dopaminergic pathways in the human brain are involved in both physiological and behavioral processes including movement, cognition, executive functions, reward, motivation, and neuroendocrine control. Each pathway is a set of projection neurons, consisting of individual dopaminergic neurons.
Affective neuroscience is the study of how the brain processes emotions. This field combines neuroscience with the psychological study of personality, emotion, and mood. The basis of emotions and what emotions are remains an issue of debate within the field of affective neuroscience.
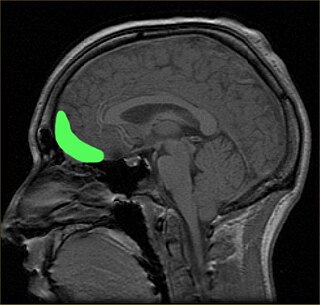
The orbitofrontal cortex (OFC) is a prefrontal cortex region in the frontal lobes of the brain which is involved in the cognitive process of decision-making. In non-human primates it consists of the association cortex areas Brodmann area 11, 12 and 13; in humans it consists of Brodmann area 10, 11 and 47.
Joseph E. LeDoux is an American neuroscientist whose research is primarily focused on survival circuits, including their impacts on emotions such as fear and anxiety. LeDoux is the Henry and Lucy Moses Professor of Science at New York University, and director of the Emotional Brain Institute, a collaboration between NYU and New York State with research sites at NYU and the Nathan Kline Institute for Psychiatric Research in Orangeburg, New York. He is also the lead singer and songwriter in the band The Amygdaloids.

The reward system is a group of neural structures responsible for incentive salience, associative learning, and positively-valenced emotions, particularly ones involving pleasure as a core component. Reward is the attractive and motivational property of a stimulus that induces appetitive behavior, also known as approach behavior, and consummatory behavior. A rewarding stimulus has been described as "any stimulus, object, event, activity, or situation that has the potential to make us approach and consume it is by definition a reward". In operant conditioning, rewarding stimuli function as positive reinforcers; however, the converse statement also holds true: positive reinforcers are rewarding.
Coherence therapy is a system of psychotherapy based in the theory that symptoms of mood, thought and behavior are produced coherently according to the person's current mental models of reality, most of which are implicit and unconscious. It was founded by Bruce Ecker and Laurel Hulley in the 1990s. It has been considered among the most well respected postmodern/constructivist therapies.

The ventromedial prefrontal cortex (vmPFC) is a part of the prefrontal cortex in the mammalian brain. The ventral medial prefrontal is located in the frontal lobe at the bottom of the cerebral hemispheres and is implicated in the processing of risk and fear, as it is critical in the regulation of amygdala activity in humans. It also plays a role in the inhibition of emotional responses, and in the process of decision-making and self-control. It is also involved in the cognitive evaluation of morality.

The basolateral amygdala, or basolateral complex, consists of the lateral, basal and accessory-basal nuclei of the amygdala. The lateral nuclei receives the majority of sensory information, which arrives directly from the temporal lobe structures, including the hippocampus and primary auditory cortex. The basolateral amygdala also receives dense neuromodulatory inputs from ventral tegmental area (VTA), locus coeruleus (LC), and basal forebrain, whose integrity are important for associative learning. The information is then processed by the basolateral complex and is sent as output to the central nucleus of the amygdala. This is how most emotional arousal is formed in mammals.
The Intercalatedcells of the amygdala are GABAergic neurons situated between the basolateral and central nuclei of the amygdala that play a significant role in inhibitory control over the amygdala. They regulate amygdala-dependent emotional processing like fear memory and social behavior. Their function has been best studied with selective ITC ablation which impairs fear extinction, fear generalization, and social behavior. Studies have begun to recognize that ITC clusters may be implicated in reward, addiction, and withdrawal circuits given their heavy expression of dopamine and opioid receptors.
Memory consolidation is a category of processes that stabilize a memory trace after its initial acquisition. A memory trace is a change in the nervous system caused by memorizing something. Consolidation is distinguished into two specific processes. The first, synaptic consolidation, which is thought to correspond to late-phase long-term potentiation, occurs on a small scale in the synaptic connections and neural circuits within the first few hours after learning. The second process is systems consolidation, occurring on a much larger scale in the brain, rendering hippocampus-dependent memories independent of the hippocampus over a period of weeks to years. Recently, a third process has become the focus of research, reconsolidation, in which previously consolidated memories can be made labile again through reactivation of the memory trace.
The management of traumatic memories is important when treating mental health disorders such as post traumatic stress disorder. Traumatic memories can cause life problems even to individuals who do not meet the diagnostic criteria for a mental health disorder. They result from traumatic experiences, including natural disasters such as earthquakes and tsunamis; violent events such as kidnapping, terrorist attacks, war, domestic abuse and rape. Traumatic memories are naturally stressful in nature and emotionally overwhelm people's existing coping mechanisms.

Memory is the faculty of the mind by which data or information is encoded, stored, and retrieved when needed. It is the retention of information over time for the purpose of influencing future action. If past events could not be remembered, it would be impossible for language, relationships, or personal identity to develop. Memory loss is usually described as forgetfulness or amnesia.
Many experiments have been done to find out how the brain interprets stimuli and how animals develop fear responses. The emotion, fear, has been hard-wired into almost every individual, due to its vital role in the survival of the individual. Researchers have found that fear is established unconsciously and that the amygdala is involved with fear conditioning.
Nadine Gogolla is a Research Group Leader at the Max Planck Institute of Neurobiology in Martinsried, Germany as well as an Associate Faculty of the Graduate School for Systemic Neuroscience. Gogolla investigates the neural circuits underlying emotion to understand how the brain integrates external cues, feeling states, and emotions to make calculated behavioral decisions. Gogolla is known for her discovery using machine learning and two-photon microscopy to classify mouse facial expressions into emotion-like categories and correlate these facial expressions with neural activity in the insular cortex.
Moriel Zelikowsky is a neuroscientist at University of Utah School of Medicine. Her laboratory studies the brain circuits and neural mechanisms underlying stress, fear, and social behavior. Her previous work includes fear and the hippocampus, and the role of neuropeptide Tac2 in social isolation.
References
- ↑ Yogis, Jaimal (2013-01-08). The Fear Project: What Our Most Primal Emotion Taught Me About Survival, Success, Surfing . . . and Love. Rodale. pp. 18–20. ISBN 9781609611767 . Retrieved 10 July 2013.
- ↑ "Neuroscience Department - Schiller Lab Home". Mount Sinai School of Medicine. Retrieved 10 July 2013.[ non-primary source needed ]
- 1 2 Hall, Stephen S. (June 17, 2013). "Neuroscientist Daniela Schiller is Researching Ways that Bad Memories Can be Made Less Fearsome". MIT Technology Review. Archived from the original on June 19, 2013. Retrieved 10 July 2013.
- ↑ Alleyne, Richard (December 10, 2009). "Trauma and fear to be erased from your mind". The Daily Telegraph . Archived from the original on December 12, 2009. Retrieved 10 July 2013.
- ↑ Reardon, Sara (April 13, 2012). "Drug-free therapy makes addicts 'forget' addiction". New Scientist . Retrieved 10 July 2013.
- ↑ Specter, Michael (19 May 2014). "Partial Recall". The New Yorker. Retrieved 27 February 2015.
- ↑ Heydarpour, Roja (6 March 2007). "INK; A Band of Scientists Who Really Are a Band". The New York Times. p. 2. Retrieved 10 July 2013.
- ↑ https://supersmallmusic.com.
{{cite web}}: Missing or empty|title=(help) - ↑ "The Esther A. & and Joseph Klingenstein Fund, Inc". www.klingfund.org. Retrieved 2020-06-10.
- ↑ "Kavli Frontiers Home". www.nasonline.org.
- ↑ "Daniela Schiller | Blavatnik Awards for Young Scientists". blavatnikawards.org.
- ↑ "Fulbright Postdoctoral Fellowship | Fulbright". www.fulbright.org.il.
- ↑ "Schiller Lab | Neuroscience Labs - Icahn School of Medicine". labs.neuroscience.mssm.edu.
- ↑ Schiller, D.; Levy, I.; Niv, Y.; LeDoux, J. E.; Phelps, E. A. (5 November 2008). "From Fear to Safety and Back: Reversal of Fear in the Human Brain". Journal of Neuroscience. 28 (45): 11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008 . PMC 3844784 . PMID 18987188.[ non-primary source needed ]
- ↑ Schiller, Daniela; Delgado, Mauricio R. (June 2010). "Overlapping neural systems mediating extinction, reversal and regulation of fear". Trends in Cognitive Sciences. 14 (6): 268–276. doi:10.1016/j.tics.2010.04.002. PMC 3848321 . PMID 20493762.[ non-primary source needed ]
- ↑ Li, Jian; Schiller, Daniela; Schoenbaum, Geoffrey; Phelps, Elizabeth A; Daw, Nathaniel D (11 September 2011). "Differential roles of human striatum and amygdala in associative learning". Nature Neuroscience. 14 (10): 1250–1252. doi:10.1038/nn.2904. PMC 3268261 . PMID 21909088.[ non-primary source needed ]
- ↑ Homan, Philipp; Levy, Ifat; Feltham, Eric; Gordon, Charles; Hu, Jingchu; Li, Jian; Pietrzak, Robert H.; Southwick, Steven; Krystal, John H.; Harpaz-Rotem, Ilan; Schiller, Daniela (21 January 2019). "Neural computations of threat in the aftermath of combat trauma". Nature Neuroscience. 22 (3): 470–476. doi:10.1038/s41593-018-0315-x. PMC 6829910 . PMID 30664770.[ non-primary source needed ]
- ↑ Collins, K. A.; Mendelsohn, A.; Cain, C. K.; Schiller, D. (29 October 2014). "Taking Action in the Face of Threat: Neural Synchronization Predicts Adaptive Coping". Journal of Neuroscience. 34 (44): 14733–14738. doi: 10.1523/JNEUROSCI.2152-14.2014 . PMC 4212070 . PMID 25355225.[ non-primary source needed ]
- 1 2 Haubrich, Josue; Nader, Karim (2016). "Memory Reconsolidation". Behavioral Neuroscience of Learning and Memory. Current Topics in Behavioral Neurosciences. Vol. 37. pp. 151–176. doi:10.1007/7854_2016_463. ISBN 978-3-319-78755-8. PMID 27885549.
- ↑ Nader, Karim; Schafe, Glenn E.; Le Doux, Joseph E. (17 August 2000). "Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval". Nature. 406 (6797): 722–726. Bibcode:2000Natur.406..722N. doi:10.1038/35021052. PMID 10963596. S2CID 4420637.
- ↑ Walker, Matthew P.; Brakefield, Tiffany; Allan Hobson, J.; Stickgold, Robert (October 2003). "Dissociable stages of human memory consolidation and reconsolidation". Nature. 425 (6958): 616–620. Bibcode:2003Natur.425..616W. doi:10.1038/nature01930. PMID 14534587. S2CID 4431941.
- ↑ Hupbach, A.; Gomez, R.; Hardt, O.; Nadel, L. (3 January 2007). "Reconsolidation of episodic memories: A subtle reminder triggers integration of new information". Learning & Memory. 14 (1–2): 47–53. doi:10.1101/lm.365707. PMC 1838545 . PMID 17202429.
- ↑ Monfils, M.-H.; Cowansage, K. K.; Klann, E.; LeDoux, J. E. (1 April 2009). "Extinction-Reconsolidation Boundaries: Key to Persistent Attenuation of Fear Memories". Science. 324 (5929): 951–955. Bibcode:2009Sci...324..951M. doi:10.1126/science.1167975. PMC 3625935 . PMID 19342552.
- ↑ Schiller, Daniela; Monfils, Marie-H.; Raio, Candace M.; Johnson, David C.; LeDoux, Joseph E.; Phelps, Elizabeth A. (9 December 2009). "Preventing the return of fear in humans using reconsolidation update mechanisms". Nature. 463 (7277): 49–53. doi:10.1038/nature08637. PMC 3640262 . PMID 20010606.[ non-primary source needed ]
- ↑ Chalkia, Anastasia; Schroyens, Natalie; Leng, Lu; Vanhasbroeck, Niels; Zenses, Ann-Kathrin; Van Oudenhove, Lukas; Beckers, Tom (2020-08-01). "No persistent attenuation of fear memories in humans: A registered replication of the reactivation-extinction effect". Cortex. 129: 496–509. doi:10.1016/j.cortex.2020.04.017. ISSN 0010-9452. PMC 7115861 . PMID 32580869.
- ↑ Chalkia, Anastasia; Van Oudenhove, Lukas; Beckers, Tom (2020-08-01). "Preventing the return of fear in humans using reconsolidation update mechanisms: A verification report of Schiller et al. (2010)". Cortex. 129: 510–525. doi:10.1016/j.cortex.2020.03.031. ISSN 0010-9452. PMC 7115860 . PMID 32563517.
- ↑ McIntosh, Robert D.; Chambers, Christopher D. (2020-08-01). "The three R's of scientific integrity: Replicability, reproducibility, and robustness". Cortex. 129: A4–A7. doi: 10.1016/j.cortex.2020.04.019 . ISSN 0010-9452. PMID 32563515. S2CID 219943250.
- 1 2 3 Schiller, Daniela; Ledoux, Joseph E.; Phelps, Elizabeth A. "Reply to Beckers, McIntosh and Chambers on the verification of 'preventing the return of fear using retrieval-extinction in humans'". psyarxiv.com. doi:10.31234/osf.io/jn6uw. S2CID 236798278 . Retrieved 2021-04-18.
- ↑ Rao-Ruiz, Priyanka; Rotaru, Diana C; van der Loo, Rolinka J; Mansvelder, Huibert D; Stiedl, Oliver; Smit, August B; Spijker, Sabine (11 September 2011). "Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear" (PDF). Nature Neuroscience. 14 (10): 1302–1308. doi:10.1038/nn.2907. PMID 21909089. S2CID 7756062.
- ↑ Escosteguy-Neto, Joao Carlos; Varela, Patricia; Correa-Neto, Nelson Francisco; Coelho, Laura Segismundo; Onaivi, Emmanuel S.; Santos-Junior, Jair Guilherme (April 2016). "Reconsolidation and update of morphine-associated contextual memory in mice". Neurobiology of Learning and Memory. 130: 194–201. doi:10.1016/j.nlm.2016.02.015. PMID 26948121. S2CID 41113005.
- ↑ Clem, R. L.; Huganir, R. L. (28 October 2010). "Calcium-Permeable AMPA Receptor Dynamics Mediate Fear Memory Erasure". Science. 330 (6007): 1108–1112. Bibcode:2010Sci...330.1108C. doi:10.1126/science.1195298. PMC 3001394 . PMID 21030604.
- ↑ Gräff, Johannes; Joseph, Nadine F.; Horn, Meryl E.; Samiei, Alireza; Meng, Jia; Seo, Jinsoo; Rei, Damien; Bero, Adam W.; Phan, Trongha X.; Wagner, Florence; Holson, Edward; Xu, Jinbin; Sun, Jianjun; Neve, Rachael L.; Mach, Robert H.; Haggarty, Stephen J.; Tsai, Li-Huei (January 2014). "Epigenetic Priming of Memory Updating during Reconsolidation to Attenuate Remote Fear Memories". Cell. 156 (1–2): 261–276. doi:10.1016/j.cell.2013.12.020. PMC 3986862 . PMID 24439381.
- ↑ Cofresí, Roberto U.; Lewis, Suzanne M.; Chaudhri, Nadia; Lee, Hongjoo J.; Monfils, Marie-H.; Gonzales, Rueben A. (March 2017). "Postretrieval Extinction Attenuates Alcohol Cue Reactivity in Rats". Alcoholism: Clinical and Experimental Research. 41 (3): 608–617. doi:10.1111/acer.13323. PMC 5332343 . PMID 28169439.
- ↑ Lee, Hongjoo J.; Haberman, Rebecca P.; Roquet, Rheall F.; Monfils, Marie-H. (22 January 2016). "Extinction and Retrieval + Extinction of Conditioned Fear Differentially Activate Medial Prefrontal Cortex and Amygdala in Rats". Frontiers in Behavioral Neuroscience. 9: 369. doi: 10.3389/fnbeh.2015.00369 . PMC 4722140 . PMID 26834596.
- ↑ Tedesco, Vincenzo; Roquet, Rheall F.; DeMis, John; Chiamulera, Cristiano; Monfils, Marie-H. (November 2014). "Extinction, applied after retrieval of auditory fear memory, selectively increases zinc-finger protein 268 and phosphorylated ribosomal protein S6 expression in prefrontal cortex and lateral amygdala". Neurobiology of Learning and Memory. 115: 78–85. doi: 10.1016/j.nlm.2014.08.015 . PMID 25196703.
- ↑ Auchter, Allison; Cormack, Lawrence K.; Niv, Yael; Gonzalez-Lima, Francisco; Monfils, Marie H. (24 January 2017). "Reconsolidation-Extinction Interactions in Fear Memory Attenuation: The Role of Inter-Trial Interval Variability". Frontiers in Behavioral Neuroscience. 11: 2. doi: 10.3389/fnbeh.2017.00002 . PMC 5258753 . PMID 28174526.
- ↑ Auchter, Allison M.; Shumake, Jason; Gonzalez-Lima, Francisco; Monfils, Marie H. (11 April 2017). "Preventing the return of fear using reconsolidation updating and methylene blue is differentially dependent on extinction learning". Scientific Reports. 7 (1): 46071. Bibcode:2017NatSR...746071A. doi: 10.1038/srep46071 . PMC 5387397 . PMID 28397861.
- ↑ Flavell, Charlotte R.; Barber, David J.; Lee, Jonathan L.C. (18 October 2011). "Behavioural memory reconsolidation of food and fear memories". Nature Communications. 2 (1): 504. Bibcode:2011NatCo...2..504F. doi: 10.1038/ncomms1515 . PMC 3516828 . PMID 22009036.
- 1 2 Xue, Y.-X.; Luo, Y.-X.; Wu, P.; Shi, H.-S.; Xue, L.-F.; Chen, C.; Zhu, W.-L.; Ding, Z.-B.; Bao, Y.-p.; Shi, J.; Epstein, D. H.; Shaham, Y.; Lu, L. (12 April 2012). "A Memory Retrieval-Extinction Procedure to Prevent Drug Craving and Relapse". Science. 336 (6078): 241–245. Bibcode:2012Sci...336..241X. doi:10.1126/science.1215070. PMC 3695463 . PMID 22499948.
- ↑ Ferrer Monti, Roque I.; Alfei, Joaquín M.; Mugnaini, Matías; Bueno, Adrián M.; Beckers, Tom; Urcelay, Gonzalo P.; Molina, Victor A. (17 July 2017). "A comparison of behavioral and pharmacological interventions to attenuate reactivated fear memories". Learning & Memory. 24 (8): 369–374. doi:10.1101/lm.045385.117. PMC 5516684 . PMID 28716956.
- ↑ Olshavsky, Megan E.; Song, Bryan J.; Powell, Daniel J.; Jones, Carolyn E.; Monfils, Marie-H.; Lee, Hongjoo J. (2013). "Updating appetitive memory during reconsolidation window: critical role of cue-directed behavior and amygdala central nucleus". Frontiers in Behavioral Neuroscience. 7: 186. doi: 10.3389/fnbeh.2013.00186 . PMC 3856395 . PMID 24367304.
- 1 2 Liu, Jianfeng; Zhao, Liyan; Xue, Yanxue; Shi, Jie; Suo, Lin; Luo, Yixiao; Chai, Baisheng; Yang, Chang; Fang, Qin; Zhang, Yan; Bao, Yanping; Pickens, Charles L.; Lu, Lin (December 2014). "An Unconditioned Stimulus Retrieval Extinction Procedure to Prevent the Return of Fear Memory". Biological Psychiatry. 76 (11): 895–901. doi:10.1016/j.biopsych.2014.03.027. PMC 4480632 . PMID 24813334.
- ↑ Luo, Yi-xiao; Xue, Yan-xue; Liu, Jian-feng; Shi, Hai-shui; Jian, Min; Han, Ying; Zhu, Wei-li; Bao, Yan-ping; Wu, Ping; Ding, Zeng-bo; Shen, Hao-wei; Shi, Jie; Shaham, Yavin; Lu, Lin (14 July 2015). "A novel UCS memory retrieval-extinction procedure to inhibit relapse to drug seeking". Nature Communications. 6 (1): 7675. Bibcode:2015NatCo...6.7675L. doi:10.1038/ncomms8675. PMC 4510700 . PMID 26169171.
- ↑ Ponnusamy, Ravikumar; Zhuravka, Irina; Poulos, Andrew M.; Shobe, Justin; Merjanian, Michael; Huang, Jeannie; Wolvek, David; O’Neill, Pia-Kelsey; Fanselow, Michael S. (9 May 2016). "Retrieval and Reconsolidation Accounts of Fear Extinction". Frontiers in Behavioral Neuroscience. 10: 89. doi: 10.3389/fnbeh.2016.00089 . PMC 4860411 . PMID 27242459.
- ↑ Yang, Yong; Jie, Jing; Li, Junjiao; Chen, Wei; Zheng, Xifu (November 2019). "A novel method to trigger the reconsolidation of fear memory". Behaviour Research and Therapy. 122: 103461. doi:10.1016/j.brat.2019.103461. PMID 31585344. S2CID 202249647.
- ↑ Junjiao, Li; Wei, Chen; Jingwen, Caoyang; Yanjian, Hu; Yong, Yang; Liang, Xu; Jing, Jie; Xifu, Zheng (December 2019). "Role of prediction error in destabilizing fear memories in retrieval extinction and its neural mechanisms". Cortex. 121: 292–307. doi:10.1016/j.cortex.2019.09.003. PMID 31669978. S2CID 203581809.
- ↑ Schiller, D.; Kanen, J. W.; LeDoux, J. E.; Monfils, M.-H.; Phelps, E. A. (25 November 2013). "Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement". Proceedings of the National Academy of Sciences. 110 (50): 20040–20045. Bibcode:2013PNAS..11020040S. doi: 10.1073/pnas.1320322110 . PMC 3864277 . PMID 24277809.[ non-primary source needed ]
- ↑ Thompson, Alina; Lipp, Ottmar V. (May 2017). "Extinction during reconsolidation eliminates recovery of fear conditioned to fear-irrelevant and fear-relevant stimuli". Behaviour Research and Therapy. 92: 1–10. doi:10.1016/j.brat.2017.01.017. PMID 28171767.
- ↑ Agren, T.; Engman, J.; Frick, A.; Bjorkstrand, J.; Larsson, E.-M.; Furmark, T.; Fredrikson, M. (20 September 2012). "Disruption of Reconsolidation Erases a Fear Memory Trace in the Human Amygdala". Science. 337 (6101): 1550–1552. Bibcode:2012Sci...337.1550A. doi:10.1126/science.1223006. PMID 22997340. S2CID 39399936.
- ↑ Feng, Pan; Zheng, Yong; Feng, Tingyong (June 2016). "Resting-state functional connectivity between amygdala and the ventromedial prefrontal cortex following fear reminder predicts fear extinction". Social Cognitive and Affective Neuroscience. 11 (6): 991–1001. doi: 10.1093/scan/nsw031 . PMC 4884324 . PMID 27013104.
- ↑ Agren, T; Furmark, T; Eriksson, E; Fredrikson, M (7 February 2012). "Human fear reconsolidation and allelic differences in serotonergic and dopaminergic genes". Translational Psychiatry. 2 (2): e76. doi: 10.1038/tp.2012.5 . PMC 3309551 . PMID 22832813.
- ↑ Feng, Pan; Zheng, Yong; Feng, Tingyong (18 November 2015). "Spontaneous brain activity following fear reminder of fear conditioning by using resting-state functional MRI". Scientific Reports. 5 (1): 16701. Bibcode:2015NatSR...516701F. doi:10.1038/srep16701. PMC 4649361 . PMID 26576733.
- ↑ Oyarzún, Javiera P.; Lopez-Barroso, Diana; Fuentemilla, Lluís; Cucurell, David; Pedraza, Carmen; Rodriguez-Fornells, Antoni; de Diego-Balaguer, Ruth; El-Deredy, Wael (29 June 2012). "Updating Fearful Memories with Extinction Training during Reconsolidation: A Human Study Using Auditory Aversive Stimuli". PLOS ONE. 7 (6): e38849. Bibcode:2012PLoSO...738849O. doi: 10.1371/journal.pone.0038849 . PMC 3387215 . PMID 22768048.
- ↑ Steinfurth, E. C. K.; Kanen, J. W.; Raio, C. M.; Clem, R. L.; Huganir, R. L.; Phelps, E. A. (16 June 2014). "Young and old Pavlovian fear memories can be modified with extinction training during reconsolidation in humans". Learning & Memory. 21 (7): 338–341. doi: 10.1101/lm.033589.113 . PMC 4061428 . PMID 24934333.
- ↑ Asthana, Manish Kumar; Brunhuber, Bettina; Mühlberger, Andreas; Reif, Andreas; Schneider, Simone; Herrmann, Martin J. (30 December 2015). "Preventing the Return of Fear Using Reconsolidation Update Mechanisms Depends on the Met-Allele of the Brain Derived Neurotrophic Factor Val66Met Polymorphism". International Journal of Neuropsychopharmacology. 19 (6): pyv137. doi: 10.1093/ijnp/pyv137 . PMC 4926796 . PMID 26721948.
- 1 2 Björkstrand, Johannes; Agren, Thomas; Åhs, Fredrik; Frick, Andreas; Larsson, Elna-Marie; Hjorth, Olof; Furmark, Tomas; Fredrikson, Mats (October 2016). "Disrupting Reconsolidation Attenuates Long-Term Fear Memory in the Human Amygdala and Facilitates Approach Behavior". Current Biology. 26 (19): 2690–2695. doi: 10.1016/j.cub.2016.08.022 . PMID 27568591.
- 1 2 Hu, Jingchu; Wang, Wenqing; Homan, Philipp; Wang, Penggui; Zheng, Xifu; Schiller, Daniela (11 June 2018). "Reminder duration determines threat memory modification in humans". Scientific Reports. 8 (1): 8848. Bibcode:2018NatSR...8.8848H. doi: 10.1038/s41598-018-27252-0 . PMC 5995965 . PMID 29891856.[ non-primary source needed ]
- ↑ Kitamura, Haruka; Johnston, Patrick; Johnson, Luke; Strodl, Esben (1 October 2020). "Boundary conditions of post-retrieval extinction: A direct comparison of low and high partial reinforcement" (PDF). Neurobiology of Learning and Memory. 174: 107285. doi:10.1016/j.nlm.2020.107285. PMID 32745600. S2CID 220886873.
- ↑ Jones, Carolyn E.; Monfils, Marie-H. (15 September 2016). "Post-retrieval extinction in adolescence prevents return of juvenile fear". Learning & Memory. 23 (10): 567–575. doi: 10.1101/lm.043281.116 . PMC 5026207 . PMID 27634147.
- ↑ Johnson, D. C.; Casey, B. J. (9 March 2015). "Extinction during memory reconsolidation blocks recovery of fear in adolescents". Scientific Reports. 5 (1): 8863. Bibcode:2015NatSR...5E8863J. doi: 10.1038/srep08863 . PMC 4352863 . PMID 25749583.
- ↑ Golkar, Armita; Tjaden, Cathelijn; Kindt, Merel (May 2017). "Vicarious extinction learning during reconsolidation neutralizes fear memory". Behaviour Research and Therapy. 92: 87–93. doi:10.1016/j.brat.2017.02.004. PMID 28286265.
- ↑ Agren, Thomas; Björkstrand, Johannes; Fredrikson, Mats (February 2017). "Disruption of human fear reconsolidation using imaginal and in vivo extinction". Behavioural Brain Research. 319: 9–15. doi:10.1016/j.bbr.2016.11.014. PMID 27840245. S2CID 25659581.
- ↑ Germeroth, Lisa J.; Carpenter, Matthew J.; Baker, Nathaniel L.; Froeliger, Brett; LaRowe, Steven D.; Saladin, Michael E. (1 March 2017). "Effect of a Brief Memory Updating Intervention on Smoking Behavior". JAMA Psychiatry. 74 (3): 214–223. doi: 10.1001/jamapsychiatry.2016.3148 . PMC 5930385 . PMID 28146243.
- ↑ Vermes, Joana Singer; Ayres, Ricardo; Goés, Adara Saito; Real, Natalia Del; Araújo, Álvaro Cabral; Schiller, Daniela; Neto, Francisco Lotufo; Corchs, Felipe (1 November 2020). "Targeting the reconsolidation of traumatic memories with a brief 2-session imaginal exposure intervention in post-traumatic stress disorder". Journal of Affective Disorders. 276: 487–494. doi:10.1016/j.jad.2020.06.052. PMID 32794448. S2CID 221128004.
- ↑ Björkstrand, Johannes; Agren, Thomas; Åhs, Fredrik; Frick, Andreas; Larsson, Elna-Marie; Hjorth, Olof; Furmark, Tomas; Fredrikson, Mats (May 2017). "Think twice, it's all right: Long lasting effects of disrupted reconsolidation on brain and behavior in human long-term fear". Behavioural Brain Research. 324: 125–129. doi:10.1016/j.bbr.2017.02.016. PMID 28214541. S2CID 205894307.
- ↑ Lane, R.D. and Nadel, L. (2020) Neuroscience of Enduring Change: Implications for Psychotherapy, Oxford University Press, USA[ page needed ]
- ↑ Ecker, Bruce; Hulley, Laurel; Ticic, Robin (2015). "Minding the findings: Let's not miss the message of memory reconsolidation research for psychotherapy". Behavioral and Brain Sciences. 38: e7. doi:10.1017/S0140525X14000168. PMID 26050698.
- ↑ Ecker, Bruce (2020). "Erasing Problematic Emotional Learnings". In Lane, Richard D.; Nadel, Lynn (eds.). Neuroscience of Enduring Change: Implications for Psychotherapy. Oxford University Press. pp. 273–299. doi:10.1093/oso/9780190881511.003.0011. ISBN 978-0-19-088151-1.
- ↑ Gray, Richard M.; Budden-Potts, Denise; Bourke, Frank F. (December 2017). "The Reconsolidation of Traumatic Memories (RTM) Protocol for PTSD: a Case Study" (PDF). Journal of Experiential Psychotherapy. 20 (4): 47–61.
- ↑ Khalaf, Ossama; Resch, Siegfried; Dixsaut, Lucie; Gorden, Victoire; Glauser, Liliane; Gräff, Johannes (15 June 2018). "Reactivation of recall-induced neurons contributes to remote fear memory attenuation". Science. 360 (6394): 1239–1242. Bibcode:2018Sci...360.1239K. doi: 10.1126/science.aas9875 . PMID 29903974. S2CID 49207679.
- ↑ Gräff, Johannes; Joseph, Nadine F.; Horn, Meryl E.; Samiei, Alireza; Meng, Jia; Seo, Jinsoo; Rei, Damien; Bero, Adam W.; Phan, Trongh X.; Wagner, Florence; Holson, Edward; Xu, Jinbin; Sun, Jianjun; Neve, Rachael L.; Mach, Robert H.; Haggarty, Stephen J.; Tsai, Li-Huei (January 2014). "Epigenetic Priming of Memory Updating during Reconsolidation to Attenuate Remote Fear Memories". Cell. 156 (1–2): 261–276. doi: 10.1016/j.cell.2013.12.020 . PMC 3986862 . PMID 24439381.
- ↑ Borgomaneri, Sara; Battaglia, Simone; Garofalo, Sara; Tortora, Francesco; Avenanti, Alessio; di Pellegrino, Giuseppe (30 July 2020). "State-Dependent TMS over Prefrontal Cortex Disrupts Fear-Memory Reconsolidation and Prevents the Return of Fear". Current Biology. 30 (18): 3672–3679.e4. doi: 10.1016/j.cub.2020.06.091 . PMID 32735813. S2CID 220872988.
- ↑ Phelps, Elizabeth A.; Hofmann, Stefan G. (31 July 2019). "Memory editing from science fiction to clinical practice". Nature. 572 (7767): 43–50. Bibcode:2019Natur.572...43P. doi: 10.1038/s41586-019-1433-7 . PMID 31367027.
- ↑ Agren, Thomas (June 2014). "Human reconsolidation: A reactivation and update". Brain Research Bulletin. 105: 70–82. doi:10.1016/j.brainresbull.2013.12.010. PMID 24397965. S2CID 24658799.
- ↑ Lee, Jonathan L.C.; Nader, Karim; Schiller, Daniela (July 2017). "An Update on Memory Reconsolidation Updating". Trends in Cognitive Sciences. 21 (7): 531–545. doi:10.1016/j.tics.2017.04.006. PMC 5605913 . PMID 28495311.[ non-primary source needed ]
- ↑ Chan, W. Y.; Leung, H. T.; Westbrook, R. F.; McNally, G. P. (2010). "Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning". Learning & Memory. 17 (10): 512–21. doi:10.1101/lm.1912510. PMC 2948891 . PMID 20884753.
- ↑ Ishii, Daisuke; Matsuzawa, Daisuke; Matsuda, Shingo; Tomizawa, Haruna; Sutoh, Chihiro; Shimizu, Eiji (August 2012). "No erasure effect of retrieval–extinction trial on fear memory in the hippocampus-independent and dependent paradigms". Neuroscience Letters. 523 (1): 76–81. doi:10.1016/j.neulet.2012.06.048. PMID 22750210. S2CID 20964729.
- ↑ Auber, Alessia; Tedesco, Vincenzo; Jones, Carolyn E.; Monfils, Marie-H.; Chiamulera, Christian (13 February 2013). "Post-retrieval extinction as reconsolidation interference: methodological issues or boundary conditions?". Psychopharmacology. 226 (4): 631–647. doi:10.1007/s00213-013-3004-1. PMC 3682675 . PMID 23404065.
- ↑ Chalkia, Anastasia; Schroyens, Natalie; Leng, Lu; Vanhasbroeck, Niels; Zenses, Ann-Kathrin; Van Oudenhove, Lukas; Beckers, Tom (June 2020). "No persistent attenuation of fear memories in humans: A registered replication of the reactivation-extinction effect". Cortex. 129: 496–509. doi: 10.1016/j.cortex.2020.04.017 . PMC 7115861 . PMID 32580869.
- ↑ Chalkia, Anastasia; Van Oudenhove, Lukas; Beckers, Tom (June 2020). "Preventing the return of fear in humans using reconsolidation update mechanisms: A verification report of Schiller et al. (2010)". Cortex. 129: 510–525. doi: 10.1016/j.cortex.2020.03.031 . PMC 7115860 . PMID 32563517.
- ↑ Fricchione, Jon; Greenberg, Mark S.; Spring, Justin; Wood, Nellie; Mueller-Pfeiffer, Christoph; Milad, Mohammed R.; Pitman, Roger K.; Orr, Scott P. (September 2016). "Delayed extinction fails to reduce skin conductance reactivity to fear-conditioned stimuli". Psychophysiology. 53 (9): 1343–1351. doi:10.1111/psyp.12687. PMID 27314560.
- ↑ Golkar, Armita; Bellander, Martin; Olsson, Andreas; Öhman, Arne (2012). "Are fear memories erasable?–reconsolidation of learned fear with fear-relevant and fear-irrelevant stimuli". Frontiers in Behavioral Neuroscience. 6: 80. doi: 10.3389/fnbeh.2012.00080 . PMC 3501228 . PMID 23181015.
- ↑ Klucken, Tim; Kruse, Onno; Schweckendiek, Jan; Kuepper, Yvonne; Mueller, Erik M.; Hennig, Juergen; Stark, Rudolf (June 2016). "No evidence for blocking the return of fear by disrupting reconsolidation prior to extinction learning". Cortex. 79: 112–122. doi: 10.1016/j.cortex.2016.03.015 . PMID 27111105.
- ↑ Kindt, Merel; Soeter, Marieke (January 2013). "Reconsolidation in a human fear conditioning study: A test of extinction as updating mechanism". Biological Psychology. 92 (1): 43–50. doi:10.1016/j.biopsycho.2011.09.016. PMID 21986472. S2CID 1190195.
- ↑ Meir Drexler, Shira; Merz, Christian J.; Hamacher-Dang, Tanja C.; Marquardt, Veronica; Fritsch, Nathalie; Otto, Tobias; Wolf, Oliver T. (2014). "Effects of postretrieval-extinction learning on return of contextually controlled cued fear". Behavioral Neuroscience. 128 (4): 474–481. doi:10.1037/a0036688. PMID 24841740. S2CID 2017102.
- ↑ Zimmermann, Josua; Bach, Dominik R. (16 March 2020). "Impact of a reminder/extinction procedure on threat-conditioned pupil size and skin conductance responses". Learning & Memory. 27 (4): 164–172. doi:10.1101/lm.050211.119. PMC 7079572 . PMID 32179658.
- ↑ Warren, Victor Taylor; Anderson, Kemp M.; Kwon, Cliffe; Bosshardt, Lauren; Jovanovic, Tanja; Bradley, Bekh; Norrholm, Seth Davin (September 2014). "Human fear extinction and return of fear using reconsolidation update mechanisms: The contribution of on-line expectancy ratings". Neurobiology of Learning and Memory. 113: 165–173. doi:10.1016/j.nlm.2013.10.014. PMC 4351258 . PMID 24183839.
- ↑ Luyten, L.; Beckers, T. (2017). "A preregistered, direct replication attempt of the retrieval-extinction effect in cued fear conditioning in rats". Neurobiology of Learning and Memory. 144: 208–215. doi:10.1016/j.nlm.2017.07.014. PMC 5931313 . PMID 28765085.
- ↑ Gershman, Samuel J; Monfils, Marie-H; Norman, Kenneth A; Niv, Yael (15 March 2017). "The computational nature of memory modification". eLife. 6. doi: 10.7554/eLife.23763 . PMC 5391211 . PMID 28294944.
- ↑ Cahill, Emma N.; Wood, Melissa A.; Everitt, Barry J.; Milton, Amy L. (20 December 2018). "The role of prediction error and memory destabilization in extinction of cued-fear within the reconsolidation window". Neuropsychopharmacology. 44 (10): 1762–1768. doi:10.1038/s41386-018-0299-y. PMC 6699995 . PMID 30659275.
- ↑ Cahill, Emma N.; Milton, Amy L. (17 January 2019). "Neurochemical and molecular mechanisms underlying the retrieval-extinction effect". Psychopharmacology. 236 (1): 111–132. doi:10.1007/s00213-018-5121-3. PMC 6373198 . PMID 30656364.
- ↑ Cassini, Lindsey F.; Flavell, Charlotte R.; Amaral, Olavo B.; Lee, Jonathan L.C. (16 August 2017). "On the transition from reconsolidation to extinction of contextual fear memories". Learning & Memory. 24 (9): 392–399. doi:10.1101/lm.045724.117. PMC 5580521 . PMID 28814464.
- ↑ Mendelsohn, Avi; Pine, Alex; Schiller, Daniela (January 2014). "Between Thoughts and Actions: Motivationally Salient Cues Invigorate Mental Action in the Human Brain". Neuron. 81 (1): 207–217. doi: 10.1016/j.neuron.2013.10.019 . PMID 24333054.[ non-primary source needed ]
- ↑ Reddan, Marianne Cumella; Wager, Tor Dessart; Schiller, Daniela (November 2018). "Attenuating Neural Threat Expression with Imagination". Neuron. 100 (4): 994–1005.e4. doi: 10.1016/j.neuron.2018.10.047 . PMC 6314478 . PMID 30465766.[ non-primary source needed ]
- ↑ Schiller, Daniela; Freeman, Jonathan B; Mitchell, Jason P; Uleman, James S; Phelps, Elizabeth A (8 March 2009). "A neural mechanism of first impressions". Nature Neuroscience. 12 (4): 508–514. doi:10.1038/nn.2278. PMID 19270690. S2CID 546583.
- ↑ Tavares, Rita Morais; Mendelsohn, Avi; Grossman, Yael; Williams, Christian Hamilton; Shapiro, Matthew; Trope, Yaacov; Schiller, Daniela (July 2015). "A Map for Social Navigation in the Human Brain". Neuron. 87 (1): 231–243. doi: 10.1016/j.neuron.2015.06.011 . PMC 4662863 . PMID 26139376.[ non-primary source needed ]
- ↑ Schiller, D.; Eichenbaum, H.; Buffalo, E. A.; Davachi, L.; Foster, D. J.; Leutgeb, S.; Ranganath, C. (14 October 2015). "Memory and Space: Towards an Understanding of the Cognitive Map". Journal of Neuroscience. 35 (41): 13904–13911. doi: 10.1523/JNEUROSCI.2618-15.2015 . PMC 6608181 . PMID 26468191.[ non-primary source needed ]
- ↑ Schafer, Matthew; Schiller, Daniela (October 2018). "Navigating Social Space". Neuron. 100 (2): 476–489. doi: 10.1016/j.neuron.2018.10.006 . PMC 6226014 . PMID 30359610.[ non-primary source needed ]
- ↑ "In Search of the Brain's Social Road Maps". Scientific American . February 2020.