Related Research Articles

Senescence or biological aging is the gradual deterioration of functional characteristics in living organisms. The word senescence can refer to either cellular senescence or to senescence of the whole organism. Organismal senescence involves an increase in death rates and/or a decrease in fecundity with increasing age, at least in the later part of an organism's life cycle. but it can be delayed. The 1934 discovery that calorie restriction can extend lifespans by 50% in rats, the existence of species having negligible senescence, and the existence of potentially immortal organisms such as members of the genus Hydra have motivated research into delaying senescence and thus age-related diseases. Rare human mutations can cause accelerated aging diseases.
Life extension is the concept of extending the human lifespan, either modestly through improvements in medicine or dramatically by increasing the maximum lifespan beyond its generally-settled limit of 125 years. Several researchers in the area, along with "life extensionists", "immortalists", or "longevists", postulate that future breakthroughs in tissue rejuvenation, stem cells, regenerative medicine, molecular repair, gene therapy, pharmaceuticals, and organ replacement will eventually enable humans to have indefinite lifespans through complete rejuvenation to a healthy youthful condition (agerasia). The ethical ramifications, if life extension becomes a possibility, are debated by bioethicists.

Longevity may refer to especially long-lived members of a population, whereas life expectancy is defined statistically as the average number of years remaining at a given age. For example, a population's life expectancy at birth is the same as the average age at death for all people born in the same year.
Maximum life span is a measure of the maximum amount of time one or more members of a population have been observed to survive between birth and death. The term can also denote an estimate of the maximum amount of time that a member of a given species could survive between birth and death, provided circumstances that are optimal to that member's longevity.
The free radical theory of aging states that organisms age because cells accumulate free radical damage over time. A free radical is any atom or molecule that has a single unpaired electron in an outer shell. While a few free radicals such as melanin are not chemically reactive, most biologically relevant free radicals are highly reactive. For most biological structures, free radical damage is closely associated with oxidative damage. Antioxidants are reducing agents, and limit oxidative damage to biological structures by passivating them from free radicals.
Calorie restriction mimetics (CRM), also known as energy restriction mimetics, are a hypothetical class of dietary supplements or drug candidates that would, in principle, mimic the substantial anti-aging effects that calorie restriction (CR) has on many laboratory animals and humans. CR is defined as a reduction in calorie intake of 20% to 50% without incurring malnutrition or a reduction in essential nutrients. An effective CRM would alter the key metabolic pathways involved in the effects of CR itself, leading to preserved youthful health and longer lifespan without the need to reduce food intake. The term was coined by Lane, Ingram, Roth of the National Institute on Aging in a seminal 1998 paper in the Journal of Anti-Aging Medicine, the forerunner of Rejuvenation Research. A number of genes and pathways have been shown to be involved with the actions of CR in model organisms and these represent attractive targets for drug discovery and for developing CRM. However, no effective CRM have been identified to date.
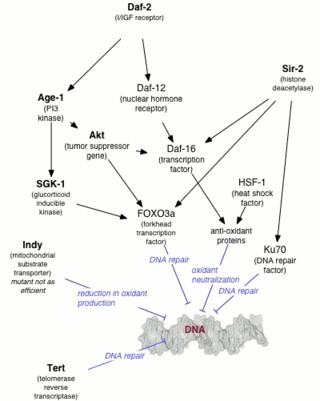
The DAF-2 gene encodes for the insulin-like growth factor 1 (IGF-1) receptor in the worm Caenorhabditis elegans. DAF-2 is part of the first metabolic pathway discovered to regulate the rate of aging. DAF-2 is also known to regulate reproductive development, resistance to oxidative stress, thermotolerance, resistance to hypoxia, and resistance to bacterial pathogens. Mutations in DAF-2 have been shown by Cynthia Kenyon to double the lifespan of the worms. In a 2007 episode of WNYC’s Radiolab, Kenyon called DAF-2 "the grim reaper gene.”

Biogerontology is the sub-field of gerontology concerned with the biological aging process, its evolutionary origins, and potential means to intervene in the process. The term "biogerontology" was coined by S. Rattan, and came in regular use with the start of the journal BIOGERONTOLOGY in 2000. It involves interdisciplinary research on the causes, effects, and mechanisms of biological aging. Biogerontologist Leonard Hayflick has said that the natural average lifespan for a human is around 92 years and, if humans do not invent new approaches to treat aging, they will be stuck with this lifespan. James Vaupel has predicted that life expectancy in industrialized countries will reach 100 for children born after the year 2000. Many surveyed biogerontologists have predicted life expectancies of more than three centuries for people born after the year 2100. Other scientists, more controversially, suggest the possibility of unlimited lifespans for those currently living. For example, Aubrey de Grey offers the "tentative timeframe" that with adequate funding of research to develop interventions in aging such as strategies for engineered negligible senescence, "we have a 50/50 chance of developing technology within about 25 to 30 years from now that will, under reasonable assumptions about the rate of subsequent improvements in that technology, allow us to stop people from dying of aging at any age". The idea of this approach is to use presently available technology to extend lifespans of currently living humans long enough for future technological progress to resolve any remaining aging-related issues. This concept has been referred to as longevity escape velocity.
Strategies for engineered negligible senescence (SENS) is a range of proposed regenerative medical therapies, either planned or currently in development, for the periodic repair of all age-related damage to human tissue. These therapies have the ultimate aim of maintaining a state of negligible senescence in patients and postponing age-associated disease. SENS was first defined by British biogerontologist Aubrey de Grey. Many mainstream scientists believe that it is a fringe theory.
The age of onset is the age at which an individual acquires, develops, or first experiences a condition or symptoms of a disease or disorder. For instance, the general age of onset for the spinal disease scoliosis is "10-15 years old," meaning that most people develop scoliosis when they are of an age between ten and fifteen years.
Enquiry into the evolution of ageing, or aging, aims to explain why a detrimental process such as ageing would evolve, and why there is so much variability in the lifespans of organisms. The classical theories of evolution suggest that environmental factors, such as predation, accidents, disease, and/or starvation, ensure that most organisms living in natural settings will not live until old age, and so there will be very little pressure to conserve genetic changes that increase longevity. Natural selection will instead strongly favor genes which ensure early maturation and rapid reproduction, and the selection for genetic traits which promote molecular and cellular self-maintenance will decline with age for most organisms.
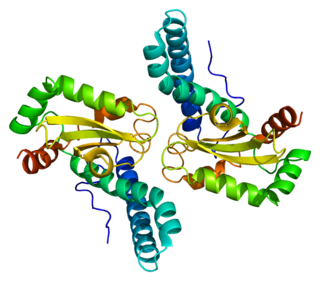
Superoxide dismutase 2, mitochondrial (SOD2), also known as manganese-dependent superoxide dismutase (MnSOD), is an enzyme which in humans is encoded by the SOD2 gene on chromosome 6. A related pseudogene has been identified on chromosome 1. Alternative splicing of this gene results in multiple transcript variants. This gene is a member of the iron/manganese superoxide dismutase family. It encodes a mitochondrial protein that forms a homotetramer and binds one manganese ion per subunit. This protein binds to the superoxide byproducts of oxidative phosphorylation and converts them to hydrogen peroxide and diatomic oxygen. Mutations in this gene have been associated with idiopathic cardiomyopathy (IDC), premature aging, sporadic motor neuron disease, and cancer.
Ageing is the process of becoming older. The term refers mainly to humans, many other animals, and fungi, whereas for example, bacteria, perennial plants and some simple animals are potentially biologically immortal. In a broader sense, ageing can refer to single cells within an organism which have ceased dividing, or to the population of a species.
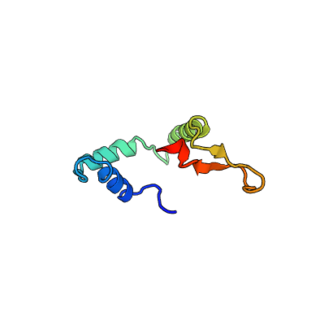
DAF-16 is the sole ortholog of the FOXO family of transcription factors in the nematode Caenorhabditis elegans. It is responsible for activating genes involved in longevity, lipogenesis, heat shock survival and oxidative stress responses. It also protects C.elegans during food deprivation, causing it to transform into a hibernation - like state, known as a Dauer. DAF-16 is notable for being the primary transcription factor required for the profound lifespan extension observed upon mutation of the insulin-like receptor DAF-2. The gene has played a large role in research into longevity and the insulin signalling pathway as it is located in C. elegans, a successful ageing model organism.
Genetics of aging is generally concerned with life extension associated with genetic alterations, rather than with accelerated aging diseases leading to reduction in lifespan.
The disposable soma theory of aging states that organisms age due to an evolutionary trade-off between growth, reproduction, and DNA repair maintenance. Formulated by Thomas Kirkwood, the disposable soma theory explains that an organism only has a limited amount of resources that it can allocate to its various cellular processes. Therefore, a greater investment in growth and reproduction would result in reduced investment in DNA repair maintenance, leading to increased cellular damage, shortened telomeres, accumulation of mutations, compromised stem cells, and ultimately, senescence. Although many models, both animal and human, have appeared to support this theory, parts of it are still controversial. Specifically, while the evolutionary trade-off between growth and aging has been well established, the relationship between reproduction and aging is still without scientific consensus, and the cellular mechanisms largely undiscovered.

The mitochondrial theory of ageing has two varieties: free radical and non-free radical. The first is one of the variants of the free radical theory of ageing. It was formulated by J. Miquel and colleagues in 1980 and was developed in the works of Linnane and coworkers (1989). The second was proposed by A. N. Lobachev in 1978.
Aging is characterized by a progressive loss of physiological integrity, leading to impaired function and increased vulnerability to death. The hallmarks of aging are the types of biochemical changes that occur in all organisms that experience biological aging and lead to a progressive loss of physiological integrity, impaired function and, eventually, death. They were first listed in a landmark paper in 2013 to conceptualize the essence of biological aging and its underlying mechanisms.

The age-1 gene is located on chromosome 2 in C.elegans. It gained attention in 1983 for its ability to induce long-lived C. elegans mutants. The age-1 mutant, first identified by Michael Klass, was reported to extend mean lifespan by over 50% at 25 °C when compared to the wild type worm (N2) in 1987 by Johnson et al. Development, metabolism, lifespan, among other processes have been associated with age-1 expression. The age-1 gene is known to share a genetic pathway with daf-2 gene that regulates lifespan in worms. Additionally, both age-1 and daf-2 mutants are dependent on daf-16 and daf-18 genes to promote lifespan extension.

Carina Kern is a geneticist whose research focuses on the biology and genetics of ageing. She is a research fellow at the Department of Genetics, Evolution and Environment in the University College London. Her work concerns the underlying causes of aging, and how they give rise to the diseases of later life. She completed her PhD at the University College London's Division of Biosciences.
References
- ↑ "Institute of Healthy Ageing (IHA)". UCL Division of Biosciences. 22 March 2019.
- ↑ "Institute of Healthy Ageing - People", accessed November 21, 2010.
- ↑ "C. elegans Ageing Laboratory", accessed November 21, 2010.
- 1 2 "David Gems Profile", accessed November 21, 2010.
- ↑ "Donald L. Riddle". nemaplex.ucdavis.edu. Retrieved 23 July 2023.
- ↑ UCL (29 January 2021). "History". UCL Division of Biosciences. Retrieved 23 July 2023.
- ↑ "Clancy, David; Gems, David; Leevers, Sally J; Oldham, Sean; Stocker, Hugo; Hafen, Ernst; Harshman, Laurence; Partridge, Linda (2001) "Extension of life span by loss of CHICO, a Drosophila insulin receptor substrate protein"". Science.
- ↑ "Selman, Colin, Lingard, Steven, Gems, David; Partridge, Linda; Withers, Dominic J. (2008) "Extended lifespan with reduced age-related pathology in insulin receptor substrate 1 null mice"". FASEB Journal.
- ↑ "Keaney, Michele; Gems, David (2003) "No increase in life span in Caenorhabditis elegans upon treatment with the superoxide dismutase mimetic EUK-8"". Free Radical Biology and Medicine.
- ↑ "Doonan, Ryan; McElwee, Joshua J.; Matthijssens, Filip; Walker, Glenda A.; Houthoofd, Koen; Back, Patricia; Matscheski, Andrea; Vanfleteren, Jacques R; Gems, David (2008) "Against the oxidative damage theory of aging: Superoxide dismutases protect against oxidative stress but have little or no effect on lifespan in C. elegans"". Genes and Development.
- ↑ "Cabreiro, Filipe; Ackerman, Daniel; Doonan, Ryan; Araiz, Caroline; Back, Patricia; Papp, Diana; Braeckman, Bart P.; Gems, David (2011) "Increased lifespan from over-expression of superoxide dismutase in C. elegans is not caused by decreased oxidative damage"". Free Radical Biology and Medicine.
- ↑ "Gems, David, Doonan, Ryan (2009) "Antioxidant defense and aging in C. elegans: Is the oxidative damage theory of aging wrong?"". Cell Cycle.
- 1 2 3 Gems, David (2022). "The hyperfunction theory: an emerging paradigm for the biology of aging". Ageing Research Reviews. 74: 101557. doi:10.1016/j.arr.2021.101557. PMC 7612201 . PMID 34990845.
- ↑ Williams, George C. (1957). "Pleiotropy, natural selection and the evolution of senescence". Evolution. 11 (4): 398–411. doi:10.2307/2406060. JSTOR 2406060.
- ↑ Blagosklonny, Mikhail V. (2006). "Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition". Cell Cycle. 5 (18): 2087–2102. doi: 10.4161/cc.5.18.3288 . PMID 17012837. S2CID 24475537.
- ↑ De Magalhães, João Pedro; Church, George M. (2005). "Genomes Optimize Reproduction: Aging as a Consequence of the Developmental Program". Physiology. 20 (4): 252–259. doi:10.1152/physiol.00010.2005. PMID 16024513.
- ↑ Gems, David; de la Guardia, Yila (2013). "Alternative perspectives on aging in C. elegans: reactive oxygen species or hyperfunction?". Antioxidants and Redox Signaling. 19 (3): 321–329. doi:10.1089/ars.2012.4840. PMC 5395017 . PMID 22870907.
- ↑ de la Guardia, Yila; Gilliat, Ann F.; Hellberg, Josephine; Rennert, Peter; Cabreiro, Filipe; Gems, David (2016). "Run-on of germline apoptosis promotes gonad senescence in C. Elegans". Oncotarget. 7 (26): 39082–39096. doi:10.18632/oncotarget.9681. PMC 5129915 . PMID 27256978.
- ↑ Wang, Hongyuan; et al. (2018). "A parthenogenetic quasi-program causes teratoma-like tumors during aging in wild-type C. elegans". npj Aging and Mechanisms of Disease. 4: 6. doi:10.1038/s41514-018-0025-3. PMC 5998035 . PMID 29928508.
- ↑ Ezcurra, Marina; et al. (2018). "C. elegans eats its own intestine to make yolk leading to multiple senescent pathologies". Current Biology. 28 (16): 2544–2556.e5. doi:10.1016/j.cub.2018.06.035. PMC 6108400 . PMID 30100339.
- ↑ Gems, David; Partridge, Linda (2013). "Genetics of longevity in model organisms: debates and paradigm shifts". Annual Review of Physiology. 75: 621–644. doi:10.1146/annurev-physiol-030212-183712. PMID 23190075.
- ↑ Gems, David; de Magalhães, João Pedro (2021). "The hoverfly and the wasp: A critique of the hallmarks of aging as a paradigm". Ageing Research Reviews. 70: 101407. doi:10.1016/j.arr.2021.101407. PMC 7611451 . PMID 34271186.
- ↑ Gems, David; Kern, Carina C. (2022). "Is 'cellular senescence' a misnomer?". GeroScience. 44 (5): 2461–2469. doi:10.1007/s11357-022-00652-x. PMC 9768054 . PMID 36068483.
- ↑ doi : 10.1016/j.arr.2019.01.008
- ↑ Gems, David; Kern, Carina C.; Nour, Joseph; Ezcurra, Marina (2021). "Reproductive Suicide: Similar Mechanisms of Aging in C. Elegans and Pacific Salmon". Frontiers in Cell and Developmental Biology. 9: 688788. doi: 10.3389/fcell.2021.688788 . PMC 8430333 . PMID 34513830.
- ↑ Kern, Carina C.; Gems, David (2022). "Semelparous Death as one Element of Iteroparous Aging Gone Large". Frontiers in Genetics. 13: 880343. doi: 10.3389/fgene.2022.880343 . PMC 9218716 . PMID 35754809.
- ↑ Gems, David (2003). "Is more life always better? Problems arising from the new biology of aging". Hastings Center Report. doi:10.2307/3528378. JSTOR 3528378.
- ↑ Gems, David (2015). "The aging-disease false dichotomy: Understanding senescence as pathology". Frontiers in Genetics. 6: 212. doi: 10.3389/fgene.2015.00212 . PMC 4468941 . PMID 26136770.