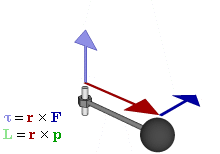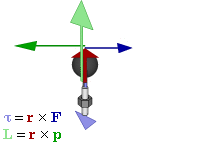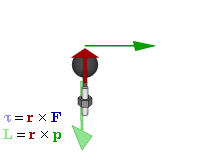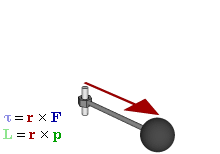
In physics and mechanics, torque is the rotational equivalent of linear force. It is also referred to as the moment, moment of force, rotational force or turning effect, depending on the field of study. The concept originated with the studies by Archimedes of the usage of levers. Just as a linear force is a push or a pull, a torque can be thought of as a twist to an object around a specific axis. Another definition of torque is the product of the magnitude of the force and the perpendicular distance of the line of action of a force from the axis of rotation. The symbol for torque is typically , the lowercase Greek letter tau. When being referred to as moment of force, it is commonly denoted by M.

In celestial mechanics, the Roche limit, also called Roche radius, is the distance from a celestial body within which a second celestial body, held together only by its own force of gravity, will disintegrate due to the first body's tidal forces exceeding the second body's gravitational self-attraction. Inside the Roche limit, orbiting material disperses and forms rings, whereas outside the limit material tends to coalesce. The Roche radius depends on the radius of the first body and on the ratio of the bodies' densities.

An ideal Fermi gas is a state of matter which is an ensemble of many non-interacting fermions. Fermions are particles that obey Fermi–Dirac statistics, like electrons, protons, and neutrons, and, in general, particles with half-integer spin. These statistics determine the energy distribution of fermions in a Fermi gas in thermal equilibrium, and is characterized by their number density, temperature, and the set of available energy states. The model is named after the Italian physicist Enrico Fermi.

In thermodynamics and solid state physics, the Debye model is a method developed by Peter Debye in 1912 for estimating the phonon contribution to the specific heat in a solid. It treats the vibrations of the atomic lattice (heat) as phonons in a box, in contrast to the Einstein model, which treats the solid as many individual, non-interacting quantum harmonic oscillators. The Debye model correctly predicts the low temperature dependence of the heat capacity, which is proportional to – the Debye T3 law. Just like the Einstein model, it also recovers the Dulong–Petit law at high temperatures. But due to simplifying assumptions, its accuracy suffers at intermediate temperatures.

In physics, a coupling constant or gauge coupling parameter, is a number that determines the strength of the force exerted in an interaction. Originally, the coupling constant related the force acting between two static bodies to the "charges" of the bodies divided by the distance squared, , between the bodies: for Newton’s gravity and for electrostatic. This description remains valid in modern physics for linear theories with static bodies and massless force carriers.
The DLVO theory explains the aggregation of aqueous dispersions quantitatively and describes the force between charged surfaces interacting through a liquid medium. It combines the effects of the van der Waals attraction and the electrostatic repulsion due to the so-called double layer of counterions. The electrostatic part of the DLVO interaction is computed in the mean field approximation in the limit of low surface potentials - that is when the potential energy of an elementary charge on the surface is much smaller than the thermal energy scale, . For two spheres of radius each having a charge separated by a center-to-center distance in a fluid of dielectric constant containing a concentration of monovalent ions, the electrostatic potential takes the form of a screened-Coulomb or Yukawa potential,

In general relativity, Schwarzschild geodesics describe the motion of particles of infinitesimal mass in the gravitational field of a central fixed mass . Schwarzschild geodesics have been pivotal in the validation of Einstein's theory of general relativity. For example, they provide accurate predictions of the anomalous precession of the planets in the Solar System, and of the deflection of light by gravity.
The Compton wavelength is a quantum mechanical property of a particle. The Compton wavelength of a particle is equal to the wavelength of a photon whose energy is the same as the mass of that particle. It was introduced by Arthur Compton in his explanation of the scattering of photons by electrons.
For the majority of numbered asteroids, almost nothing is known apart from a few physical parameters and orbital elements and some physical characteristics are often only estimated. The physical data is determined by making certain standard assumptions.
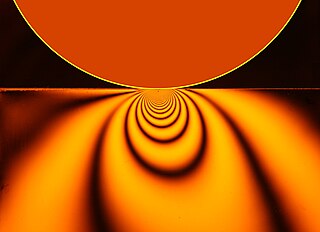
Contact mechanics is the study of the deformation of solids that touch each other at one or more points. A central distinction in contact mechanics is between stresses acting perpendicular to the contacting bodies' surfaces and frictional stresses acting tangentially between the surfaces. This page focuses mainly on the normal direction, i.e. on frictionless contact mechanics. Frictional contact mechanics is discussed separately. Normal stresses are caused by applied forces and by the adhesion present on surfaces in close contact even if they are clean and dry.

Diffusiophoresis is the spontaneous motion of colloidal particles or molecules in a fluid, induced by a concentration gradient of a different substance. In other words, it is motion of one species, A, in response to a concentration gradient in another species, B. Typically, A is colloidal particles which are in aqueous solution in which B is a dissolved salt such as sodium chloride, and so the particles of A are much larger than the ions of B. But both A and B could be polymer molecules, and B could be a small molecule. For example, concentration gradients in ethanol solutions in water move 1 μm diameter colloidal particles with diffusiophoretic velocities of order 0.1 to 1 μm/s, the movement is towards regions of the solution with lower ethanol concentration. Both species A and B will typically be diffusing but diffusiophoresis is distinct from simple diffusion: in simple diffusion a species A moves down a gradient in its own concentration.
The Thomas–Fermi (TF) model, named after Llewellyn Thomas and Enrico Fermi, is a quantum mechanical theory for the electronic structure of many-body systems developed semiclassically shortly after the introduction of the Schrödinger equation. It stands separate from wave function theory as being formulated in terms of the electronic density alone and as such is viewed as a precursor to modern density functional theory. The Thomas–Fermi model is correct only in the limit of an infinite nuclear charge. Using the approximation for realistic systems yields poor quantitative predictions, even failing to reproduce some general features of the density such as shell structure in atoms and Friedel oscillations in solids. It has, however, found modern applications in many fields through the ability to extract qualitative trends analytically and with the ease at which the model can be solved. The kinetic energy expression of Thomas–Fermi theory is also used as a component in more sophisticated density approximation to the kinetic energy within modern orbital-free density functional theory.
Disjoining pressure, in surface chemistry, according to an IUPAC definition, arises from an attractive interaction between two surfaces. For two flat and parallel surfaces, the value of the disjoining pressure can be calculated as the derivative of the Gibbs energy of interaction per unit area in respect to distance. There is also a related concept of disjoining force, which can be viewed as disjoining pressure times the surface area of the interacting surfaces.
The Grey atmosphere is a useful set of approximations made for radiative transfer applications in studies of stellar atmospheres based on the simplification that the absorption coefficient of matter within the atmosphere is constant for all frequencies of incident radiation.
Magnets exert forces and torques on each other due to the rules of electromagnetism. The forces of attraction field of magnets are due to microscopic currents of electrically charged electrons orbiting nuclei and the intrinsic magnetism of fundamental particles that make up the material. Both of these are modeled quite well as tiny loops of current called magnetic dipoles that produce their own magnetic field and are affected by external magnetic fields. The most elementary force between magnets, therefore, is the magnetic dipole–dipole interaction. If all of the magnetic dipoles that make up two magnets are known then the net force on both magnets can be determined by summing up all these interactions between the dipoles of the first magnet and that of the second.

The feet of geckos have a number of specializations. Their surfaces can adhere to any type of material with the exception of Teflon (PTFE). This phenomenon can be explained with three elements:

Double layer forces occur between charged objects across liquids, typically water. This force acts over distances that are comparable to the Debye length, which is on the order of one to a few tenths of nanometers. The strength of these forces increases with the magnitude of the surface charge density. For two similarly charged objects, this force is repulsive and decays exponentially at larger distances, see figure. For unequally charged objects and eventually at shorted distances, these forces may also be attractive. The theory due to Derjaguin, Landau, Verwey, and Overbeek (DLVO) combines such double layer forces together with Van der Waals forces in order to estimate the actual interaction potential between colloidal particles.
Thomas–Fermi screening is a theoretical approach to calculate the effects of electric field screening by electrons in a solid. It is a special case of the more general Lindhard theory; in particular, Thomas–Fermi screening is the limit of the Lindhard formula when the wavevector is much smaller than the fermi wavevector, i.e. the long-distance limit. It is named after Llewellyn Thomas and Enrico Fermi.
A depletion force is an effective attractive force that arises between large colloidal particles that are suspended in a dilute solution of depletants, which are smaller solutes that are preferentially excluded from the vicinity of the large particles. One of the earliest reports of depletion forces that lead to particle coagulation is that of Bondy, who observed the separation or 'creaming' of rubber latex upon addition of polymer depletant molecules to solution. More generally, depletants can include polymers, micelles, osmolytes, ink, mud, or paint dispersed in a continuous phase.
In condensed matter physics and physical chemistry, the Lifshitz theory of van der Waals forces, sometimes called the macroscopic theory of van der Waals forces, is a method proposed by Evgeny Mikhailovich Lifshitz in 1954 for treating van der Waals forces between bodies which does not assume pairwise additivity of the individual intermolecular forces; that is to say, the theory takes into account the influence of neighboring molecules on the interaction between every pair of molecules located in the two bodies, rather than treating each pair independently.




























