Related Research Articles

Skin is the layer of usually soft, flexible outer tissue covering the body of a vertebrate animal, with three main functions: protection, regulation, and sensation.

The integumentary system is the set of organs forming the outermost layer of an animal's body. It comprises the skin and its appendages, which act as a physical barrier between the external environment and the internal environment that it serves to protect and maintain the body of the animal. Mainly it is the body's outer skin.

A fibroblast is a type of biological cell that synthesizes the extracellular matrix and collagen, produces the structural framework (stroma) for animal tissues, and plays a critical role in wound healing. Fibroblasts are the most common cells of connective tissue in animals.

A scar is an area of fibrous tissue that replaces normal skin after an injury. Scars result from the biological process of wound repair in the skin, as well as in other organs, and tissues of the body. Thus, scarring is a natural part of the healing process. With the exception of very minor lesions, every wound results in some degree of scarring. An exception to this are animals with complete regeneration, which regrow tissue without scar formation.

Fibronectin is a high-molecular weight glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins called integrins. It is approved for marketing as a topical solution in India by Central Drugs Standard Control organization in 2020 under the brand name FIBREGA for chronic wounds. Fibronectin also binds to other extracellular matrix proteins such as collagen, fibrin, and heparan sulfate proteoglycans.

In biology, the extracellular matrix (ECM), is a network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM.
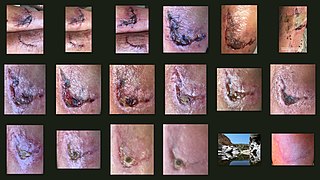
Wound healing refers to a living organism's replacement of destroyed or damaged tissue by newly produced tissue.

The dermis or corium is a layer of skin between the epidermis and subcutaneous tissues, that primarily consists of dense irregular connective tissue and cushions the body from stress and strain. It is divided into two layers, the superficial area adjacent to the epidermis called the papillary region and a deep thicker area known as the reticular dermis. The dermis is tightly connected to the epidermis through a basement membrane. Structural components of the dermis are collagen, elastic fibers, and extrafibrillar matrix. It also contains mechanoreceptors that provide the sense of touch and thermoreceptors that provide the sense of heat. In addition, hair follicles, sweat glands, sebaceous glands, apocrine glands, lymphatic vessels, nerves and blood vessels are present in the dermis. Those blood vessels provide nourishment and waste removal for both dermal and epidermal cells.

Fibrosis, also known as fibrotic scarring, is a pathological wound healing in which connective tissue replaces normal parenchymal tissue to the extent that it goes unchecked, leading to considerable tissue remodelling and the formation of permanent scar tissue.
Stromal cells, or mesenchymal stromal cells, are differentiating cells found in abundance within bone marrow but can also be seen all around the body. Stromal cells can become connective tissue cells of any organ, for example in the uterine mucosa (endometrium), prostate, bone marrow, lymph node and the ovary. They are cells that support the function of the parenchymal cells of that organ. The most common stromal cells include fibroblasts and pericytes. The term stromal comes from Latin stromat-, "bed covering", and Ancient Greek στρῶμα, strôma, "bed".

A myofibroblast is a cell phenotype that was first described as being in a state between a fibroblast and a smooth muscle cell.

Dermatopontin also known as tyrosine-rich acidic matrix protein (TRAMP) is a protein that in humans is encoded by the DPT gene. Dermatopontin is a 22-kDa protein of the noncollagenous extracellular matrix (ECM) estimated to comprise 12 mg/kg of wet dermis weight. To date, homologues have been identified in five different mammals and 12 different invertebrates with multiple functions. In vertebrates, the primary function of dermatopontin is a structural component of the ECM, cell adhesion, modulation of TGF-β activity and cellular quiescence). It also has pathological involvement in heart attacks and decreased expression in leiomyoma and fibrosis. In invertebrate, dermatopontin homologue plays a role in hemagglutination, cell-cell aggregation, and expression during parasite infection.

Artificial skin is a collagen scaffold that induces regeneration of skin in mammals such as humans. The term was used in the late 1970s and early 1980s to describe a new treatment for massive burns. It was later discovered that treatment of deep skin wounds in adult animals and humans with this scaffold induces regeneration of the dermis. It has been developed commercially under the name Integra and is used in massively burned patients, during plastic surgery of the skin, and in treatment of chronic skin wounds.

In medicine, desmoplasia is the growth of fibrous connective tissue. It is also called a desmoplastic reaction to emphasize that it is secondary to an insult. Desmoplasia may occur around a neoplasm, causing dense fibrosis around the tumor, or scar tissue (adhesions) within the abdomen after abdominal surgery.
Acellular dermis is a type of biomaterial derived from processing human or animal tissues to remove cells and retain portions of the extracellular matrix (ECM). These materials are typically cell-free, distinguishing them from classical allografts and xenografts, can be integrated or incorporated into the body, and have been FDA approved for human use for more than 10 years in a wide range of clinical indications.
The dermal equivalent, also known as dermal replacement or neodermis, is an in vitro model of the dermal layer of skin. There is no specific way of forming a dermal equivalent, however the first dermal equivalent was constructed by seeding dermal fibroblasts into a collagen gel. This gel may then be allowed to contract as a model of wound contraction. This collagen gel contraction assay may be used to screen for treatments which promote or inhibit contraction and thus affect the development of a scar. Other cell types may be incorporated into the dermal equivalent to increase the complexity of the model. For example, keratinocytes may be seeded on the surface to create a skin equivalent, or macrophages may be incorporated to model the inflammatory phase of wound healing.
Mechanobiology is an emerging field of science at the interface of biology, engineering, chemistry and physics. It focuses on how physical forces and changes in the mechanical properties of cells and tissues contribute to development, cell differentiation, physiology, and disease. Mechanical forces are experienced and may be interpreted to give biological responses in cells. The movement of joints, compressive loads on the cartilage and bone during exercise, and shear pressure on the blood vessel during blood circulation are all examples of mechanical forces in human tissues. A major challenge in the field is understanding mechanotransduction—the molecular mechanisms by which cells sense and respond to mechanical signals. While medicine has typically looked for the genetic and biochemical basis of disease, advances in mechanobiology suggest that changes in cell mechanics, extracellular matrix structure, or mechanotransduction may contribute to the development of many diseases, including atherosclerosis, fibrosis, asthma, osteoporosis, heart failure, and cancer. There is also a strong mechanical basis for many generalized medical disabilities, such as lower back pain, foot and postural injury, deformity, and irritable bowel syndrome.
Tissue engineering of oral mucosa combines cells, materials and engineering to produce a three-dimensional reconstruction of oral mucosa. It is meant to simulate the real anatomical structure and function of oral mucosa. Tissue engineered oral mucosa shows promise for clinical use, such as the replacement of soft tissue defects in the oral cavity. These defects can be divided into two major categories: the gingival recessions which are tooth-related defects, and the non tooth-related defects. Non tooth-related defects can be the result of trauma, chronic infection or defects caused by tumor resection or ablation. Common approaches for replacing damaged oral mucosa are the use of autologous grafts and cultured epithelial sheets.
A Muse cell is an endogenous non-cancerous pluripotent stem cell. They reside in the connective tissue of nearly every organ including the umbilical cord, bone marrow and peripheral blood. They are collectable from commercially obtainable mesenchymal cells such as human fibroblasts, bone marrow-mesenchymal stem cells and adipose-derived stem cells. Muse cells are able to generate cells representative of all three germ layers from a single cell both spontaneously and under cytokine induction. Expression of pluripotency genes and triploblastic differentiation are self-renewable over generations. Muse cells do not undergo teratoma formation when transplanted into a host environment in vivo. This can be explained in part by their intrinsically low telomerase activity, eradicating the risk of tumorigenesis through unbridled cell proliferation. They were discovered in 2010 by Mari Dezawa and her research group. Clinical trials for acute myocardial infarction, stroke, epidermolysis bullosa, spinal cord injury, amyotrophic lateral sclerosis, acute respiratory distress syndrome (ARDS) related to novel coronavirus (SARS-CoV-2) infection, are conducted by Life Science Institute, Inc., a group company of Mitsubishi Chemical Holdings company. In february 2023, however, Mitsubishi Chemical Group decided to discontinue the development of a regenerative medicine product (CL2020) using Muse Cells. Physician-led clinical trial for neonatal hypoxic-ischemic encephalopathy was also started. The summary results of a randomized double-blind placebo-controlled clinical trial in patients with stroke was announced.
Scar free healing is the process by which significant injuries can heal without permanent damage to the tissue the injury has affected. In most healing, scars form due to the fibrosis and wound contraction, however in scar free healing, tissue is completely regenerated. During the 1990s, published research on the subject increased; it is a relatively recent term in the literature. Scar free healing occurs in foetal life but the ability progressively diminishes into adulthood. In other animals such as amphibians, however, tissue regeneration occurs, for example as skin regeneration in the adult axolotl.
References
- ↑ "Wound and Healing". Skin Science. L'Oreal. Archived from the original on 2012-04-25. Retrieved 2011-10-02.
- ↑ Darling, David (10 September 2011). "Hypodermis". Encyclopedia of Science.
- 1 2 3 Shamis, Yulia; Hewitt, Kyle J; Carlson, Mark W; Margvelashvilli, Mariam; Dong, Shumin; Kuo, Catherine K; Daheron, Laurence; Egles, Christophe; Garlick, Jonathan A (2011). "Fibroblasts derived from human embryonic stem cells direct development and repair of 3D human skin equivalents". Stem Cell Research & Therapy. 2 (1): 10. doi: 10.1186/scrt51 . PMC 3092150 . PMID 21338517.
- 1 2 3 4 5 Alberts, B.; A. Johnson; J. Lewis (2002). "Fibroblasts and Their Transformations: The Connective-Tissue Cell Family". Microbiology of the Cell (4th ed.). New York: Garland Science.
- ↑ Hinz, Boris; Phan, Sem H.; Thannickal, Victor J.; Galli, Andrea; Bochaton-Piallat, Marie-Luce; Gabbiani, Giulio (2007). "The Myofibroblast". The American Journal of Pathology. 170 (6): 1807–16. doi:10.2353/ajpath.2007.070112. PMC 1899462 . PMID 17525249.
- 1 2 3 4 Akita, Sadanori; Akino, Kozo; Imaizumi, Toshifumi; Hirano, Akiyoshi (2008). "Basic fibroblast growth factor accelerates and improves second-degree burn wound healing". Wound Repair and Regeneration. 16 (5): 635–41. doi:10.1111/j.1524-475X.2008.00414.x. PMID 19128258. S2CID 24954846.
- 1 2 3 4 5 6 Lapouge, Gaelle; Blanpain, Cédric (2008). Silberstein, Leslie (ed.). "Medical applications of epidermal stem cells". StemBook. doi: 10.3824/stembook.1.27.1 . PMID 20614607.
- ↑ Sierra, David H.; Feldman, Dale S.; Saltz, Renato; Huang, Shu (1992). "A method to determine shear adhesive strength of fibrin sealants". Journal of Applied Biomaterials. 3 (2): 147–51. doi:10.1002/jab.770030210. PMID 10147711.
- ↑ Azadani, Ali N.; Matthews, Peter B.; Ge, Liang; Shen, Ye; Jhun, Choon-Sik; Guy, T. Sloane; Tseng, Elaine E. (2009). "Mechanical Properties of Surgical Glues Used in Aortic Root Replacement". The Annals of Thoracic Surgery. 87 (4): 1154–60. doi:10.1016/j.athoracsur.2008.12.072. PMID 19324142.
- ↑ Wenger, Marco P.E.; Bozec, Laurent; Horton, Michael A.; Mesquida, Patrick (2007). "Mechanical Properties of Collagen Fibrils☆". Biophysical Journal. 93 (4): 1255–63. Bibcode:2007BpJ....93.1255W. doi:10.1529/biophysj.106.103192. PMC 1929027 . PMID 17526569.